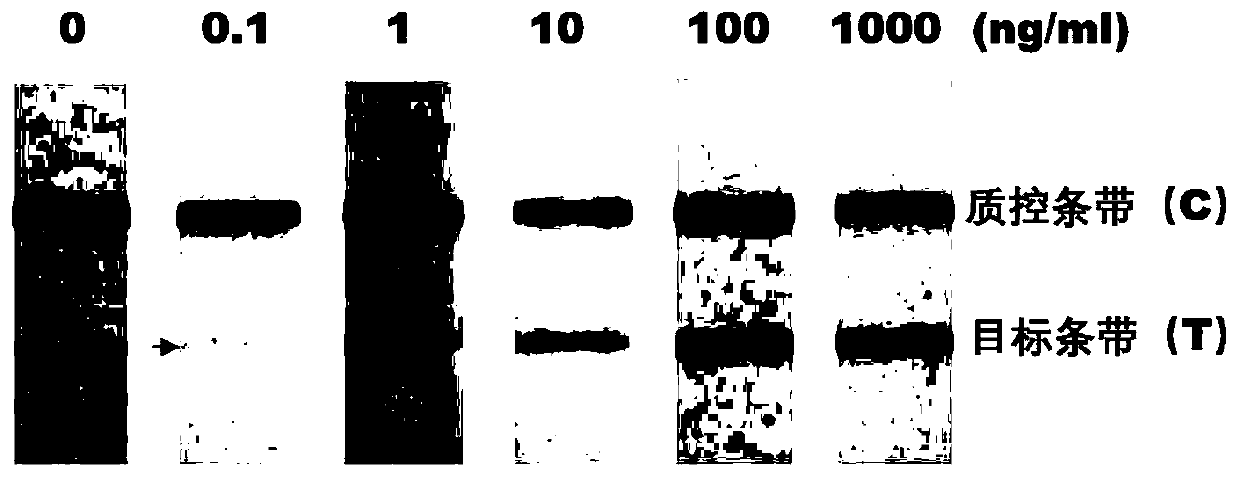
Specific epitope peptide of occludin 31kDa degradation fragment and application of specific epitope peptide
An epitope and specific technology, applied in the biological field, can solve the problem of slow rising speed, and achieve the effect of low detection limit, good specificity and improved safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、31
[0022] Example 1, Design and Synthesis of Antigenic Epitope Peptide of 31kDa Occlusin Degradation Fragment
[0023] Analysis of human Occludin protein (occlusive protein) amino acid sequence (shown in SEQ ID No.1), Occludin protein 31kDa degradation fragment amino acid sequence (321-522 shown in SEQ ID No.1), and Occludin protein 55kDa degradation fragment amino acid Sequence position, select three amino acid sequences in the amino acid sequence of the 31kDa degradation fragment of Occludin protein as candidate specific epitopes of the 31kDa degradation fragment of Occludin protein, and synthesize three polypeptides respectively, namely antigen epitope peptides T1, T2, and T3. The sequences are as follows:
[0024] Antigenic epitope peptide T1: 323-334 positions shown in SEQ ID No.1;
[0025] Antigen epitope peptide T2: No. 346-355 shown in SEQ ID No.1;
[0026] Antigen epitope peptide T3: 363-373 positions shown in SEQ ID No.1.
Embodiment 2
[0027] Embodiment 2, the preparation of antigen and antibody
[0028] The three polypeptides (antigenic epitope peptides) obtained in Example 1 were respectively linked to the carrier protein KLH (hemocyanin) to prepare three immunogenic antigens.
[0029] The three antigens were used as immune antigens respectively, and the rabbits were immunized by subcutaneous injection, and the immunization was boosted once every two weeks, a total of 4 immunizations. Blood was collected from the carotid artery to obtain antiserum, and then purified to obtain the three antibodies.
[0030] Using the ELISA method, the corresponding antigenic epitope peptide-BSA was used as the detection antigen, and the titers of the antisera containing the corresponding antibodies were tested respectively, and the results were all above 1:3000.
Embodiment 3
[0031] Embodiment 3, Western blot detection antibody specificity
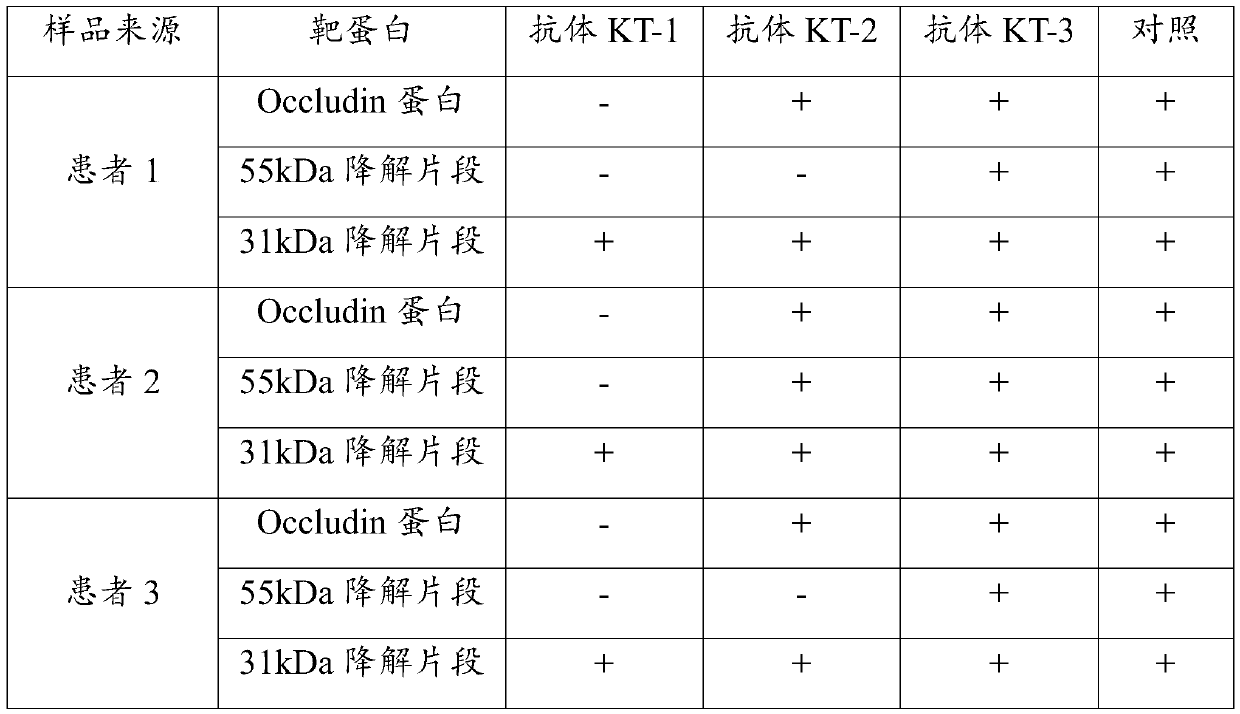
[0032] Take a sample of peripheral blood serum from 3 patients with acute ischemic stroke, perform protein electrophoresis separation and membrane transfer, and then use the three antibodies prepared in Example 2 to detect each sample separately, and use Occludin protein to detect The antibody was used as a control, and the results are shown in Table 1.
[0033] Table 1. Antibody specificity results detected by Western blot
[0034]
[0035] Note: "-" in Table 1 indicates that the result is negative, and "+" indicates that the result is positive.
[0036] The results in Table 1 show that the antibody KT-1 has the specificity of recognizing the 31kDa degraded fragment of atretin but cannot recognize the 55kDa degraded fragment of atretin and the undegraded atretin protein. Antibody KT-2 and antibody KT-3 do not have the specificity of recognizing the 31kDa degraded fragment of atretin specificity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com