Application of IncRNA in regulating polarization of macrophages in viral myocarditis
A viral myocarditis and macrophage technology, applied in the field of bioengineering, can solve the problem that the mechanism of macrophage polarization remains to be explored.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
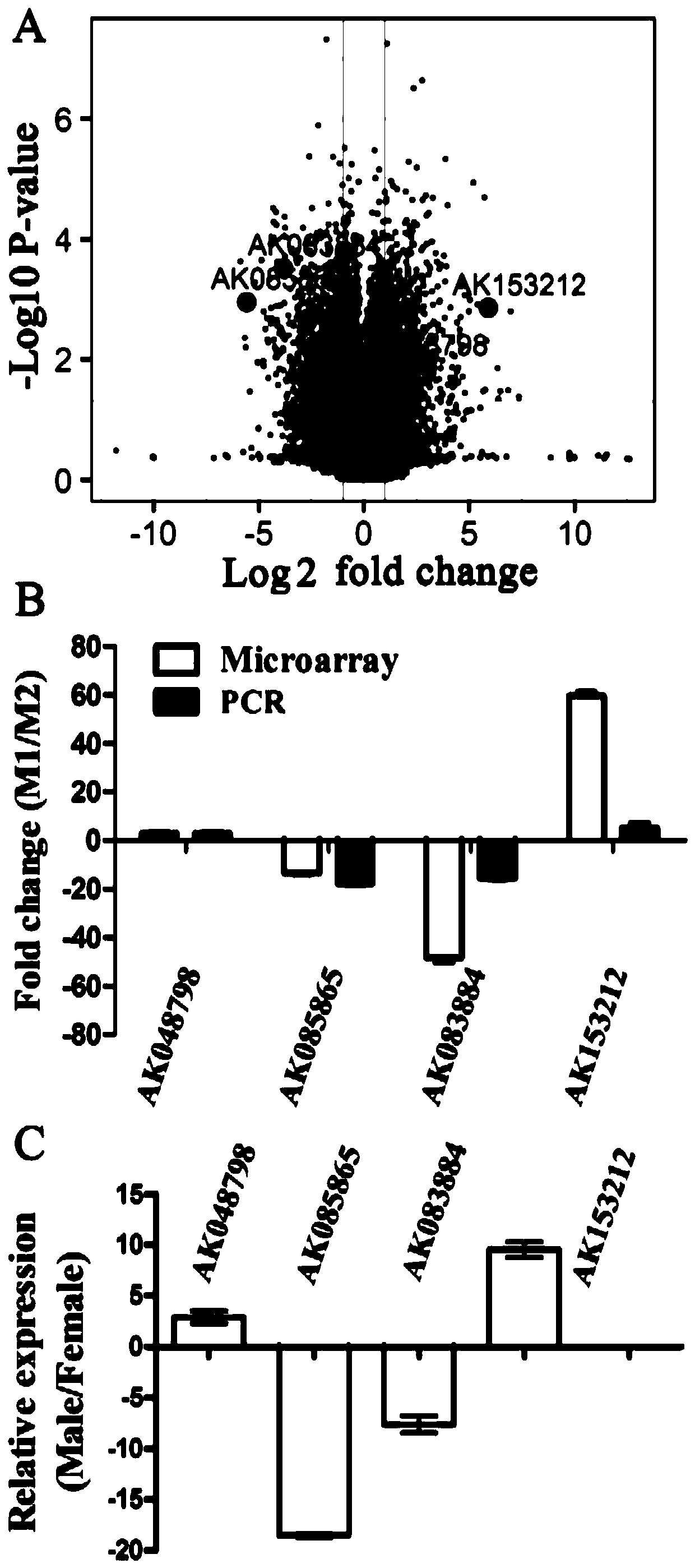
[0095] The applicants performed microarray analysis on M1 and M2 macrophages polarized in vitro to identify lncRNAs involved in macrophage polarization, and the results were as follows figure 1 As shown in A. Depend on figure 1 A shows that: when the threshold of differential expression is set to fold change ≥ 2, and P<0.05, there are 627 highly expressed lncRNAs in M1 macrophages, and 624 highly expressed lncRNAs in M2 macrophages.
[0096] According to lncRNA reverse target gene prediction combined with the adjacent gene function of differentially expressed lncRNAs, the applicant selected several lncRNAs, and used RT-qPCR to verify the chip results, and the results are shown in Figure 1B. Depend on figure 1 B: After RT-qPCR analysis, the results of 4 lncRNAs (AK048798, AK085865, AK083884, AK153212) with significant expression differences in M1 / M2 were consistent with the chip.
[0097] Previous studies have found that there are differences in the phenotypes of myocardial ...
Embodiment 2
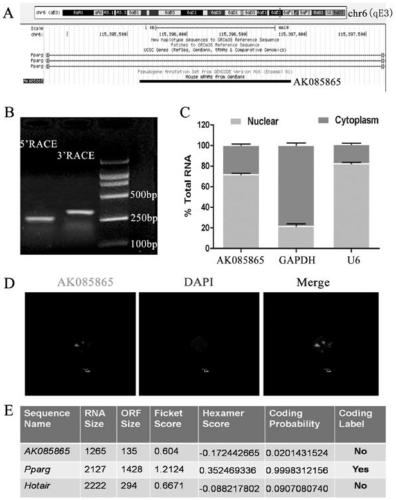
[0099] Since many lncRNAs have been proven to have positive or negative regulatory effects on their neighboring genes, the genomic location of their neighboring genes needs to be further analyzed, such as figure 2 As shown in A. AK085865 is located on chromosome 6 of the mouse and is transcribed in the second intron of the protein-coding gene PPARγ.
[0100] like figure 2 As shown in B, the applicant used Rapid Amplification of cDNA Ends (RACE) to determine the length of AK085865 to be 1266 bp.
[0101] After nucleoplasm separation, RNA was extracted for RT-qPCR detection, and the results were as follows: figure 2 C shown. Depend on figure 2 C It can be seen that about 70% of AK085865 transcripts are located in the nucleus.
[0102] like figure 2 As shown in D, fluorescence in situ hybridization (FISH) also shows that AK085865 is mainly located in the nucleus, suggesting that AK085865 may exert its biological function in the nucleus. Consistent with the definition ...
Embodiment 3
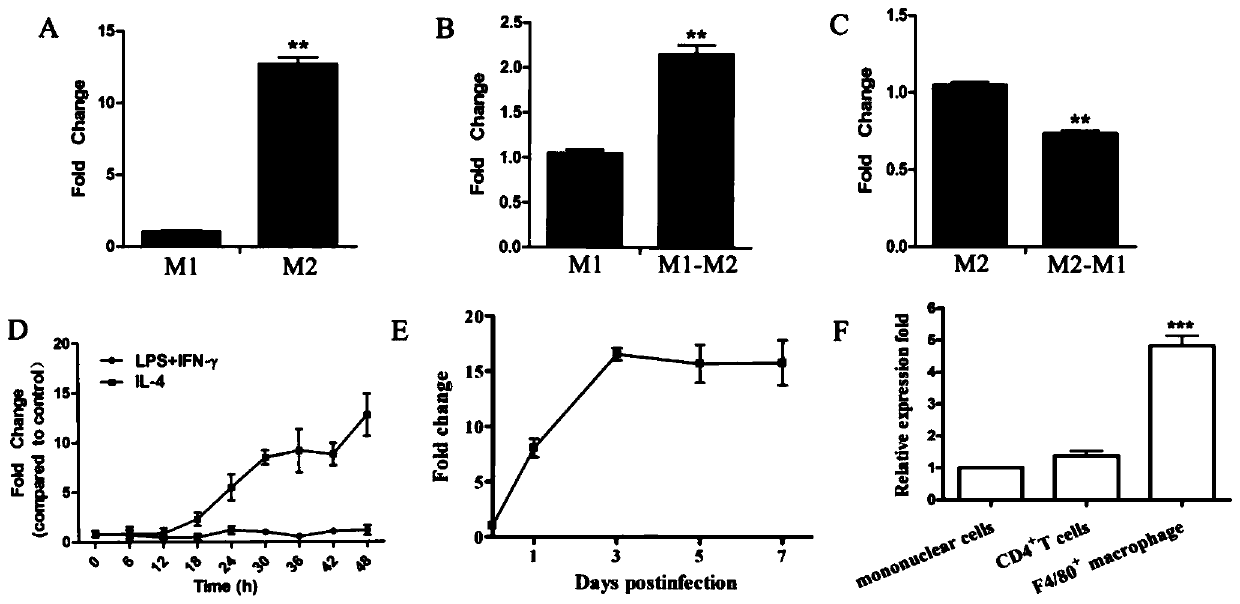
[0105] The applicant used RT-qPCR to detect the expression level of AK085865 during the polarization process of macrophages (M1 and M2), and GAPDH was used as an internal reference. The results are as follows image 3 As shown in A. Depend on image 3 A shows that the AK085865 level of M2 macrophages is significantly higher than that of M1 macrophages.
[0106] The applicant used LPS and IFN-γ to stimulate M2 macrophages or IL-4 to stimulate M1 macrophages to reverse the phenotype of macrophages to determine whether AK085865 contributes to the plasticity of macrophage polarization . M1 macrophages were cultured in fresh medium containing IL-4 for another 2 days to induce the transition from M1 to M2; M2 macrophages were cultured in fresh medium containing LPS and IFN-γ for 2 days, To induce the transformation of M2 to M1; RT-qPCR detects the level of AK085865, and the results are as follows image 3 B. image 3 C shown. Depend on image 3 B and image 3 C shows that th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com