Method for simultaneously obtaining T1 weighted imaging by using RAREVTR sequence aiming at magnetic resonance double-signal nano probe
A technology of nanoprobe and imaging method, which is applied in the direction of measurement, magnetic resonance measurement, diagnosis signal processing, etc. using nuclear magnetic resonance imaging system, and can solve problems such as the effect of aliasing signals
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
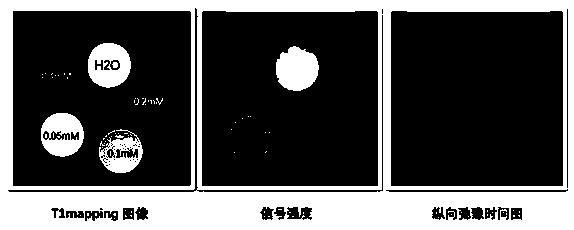
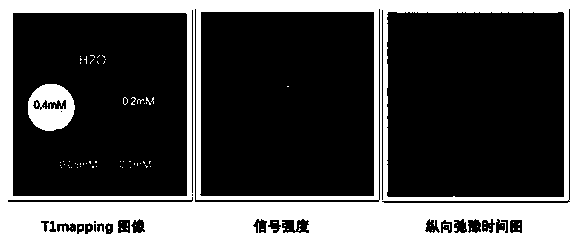
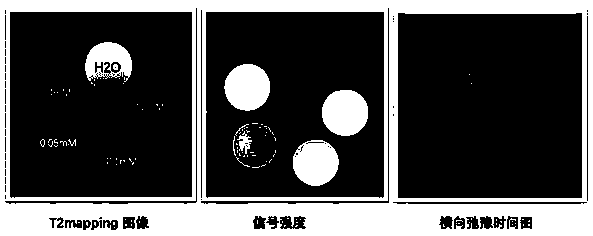
[0040] Example 1: In order to eliminate the influence of T2 aliasing signals and realize T1 weighted imaging, the maximum TR is set to be less than 1 / 5 T1 value on the basis of the original imaging sequence parameters, and T1 is the longitudinal relaxation time of the probe solution.
Embodiment 2
[0041] Embodiment 2: In order to eliminate the influence of T2 confusing signal, realize T1 weighted imaging, on the basis of original imaging sequence parameters, TE is set to be less than 1 / 5T2; Maximum TR is set to be less than 1 / 5T1 value, and described T1 is probe solution Longitudinal relaxation time, T2 is the transverse relaxation time of the probe solution.
Embodiment 3
[0042] Example 3: In order to eliminate the influence of T2 aliasing signals, T1 weighted imaging is realized. On the basis of the original imaging sequence parameters, TE is set to be less than 1 / 5T2; TR is set to multiple values, the maximum value is 4000ms, and T1 is the probe The longitudinal relaxation time of the solution, T2 is the transverse relaxation time of the probe solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com