Fusion protein with antineoplastic function and preparation method and application of fusion protein
A fusion protein and functional domain technology, which can be used in anti-tumor drugs, medical preparations containing active ingredients, fusion polypeptides, etc., and can solve problems such as limiting clinical applications.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] In this example, an integrin blocker / GnRH agonist fusion protein: LMRAP was prepared.
[0080] (1) Construction of the carrier
[0081] The LMRAP coding sequence was cloned into the EcoRI site of the plasmid vector pEE12.4 (Lonza Biologics) by means of homologous recombination, and the host bacteria were Trans1-T1 cells (Beijing Quanshijin Biology). Wherein, TAA / TGA is a stop codon.
[0082] The nucleotide sequence encoding the LMRAP protein is:
[0083]
[0084] Conversion:
[0085] Take 2 μl of the recombination product of the target gene fragment and the vector, mix gently with 50 μl of Transl-T1 competent cells, and place on ice for 30 minutes.
[0086] The mixture was heat-shocked in a water bath at 42 °C for 30 s, and then quickly transferred to ice to cool for 2 min.
[0087] Add 450 μl LB liquid medium to the mixture, shake and culture at 37°C for 1 hour, so as to facilitate the recovery of bacterial resistance.
[0088] Take 100 μl of the bacterial so...
Embodiment 2
[0105] In this example, an integrin blocker / GnRH agonist fusion protein: LMRAP-A was prepared. The steps of vector construction, stable transfection screening, production of target protein and separation and purification of target protein in this example are the same as those in Example 1.
[0106] The nucleotide sequence encoding the LMRAP-A protein is:
[0107]
Embodiment 3
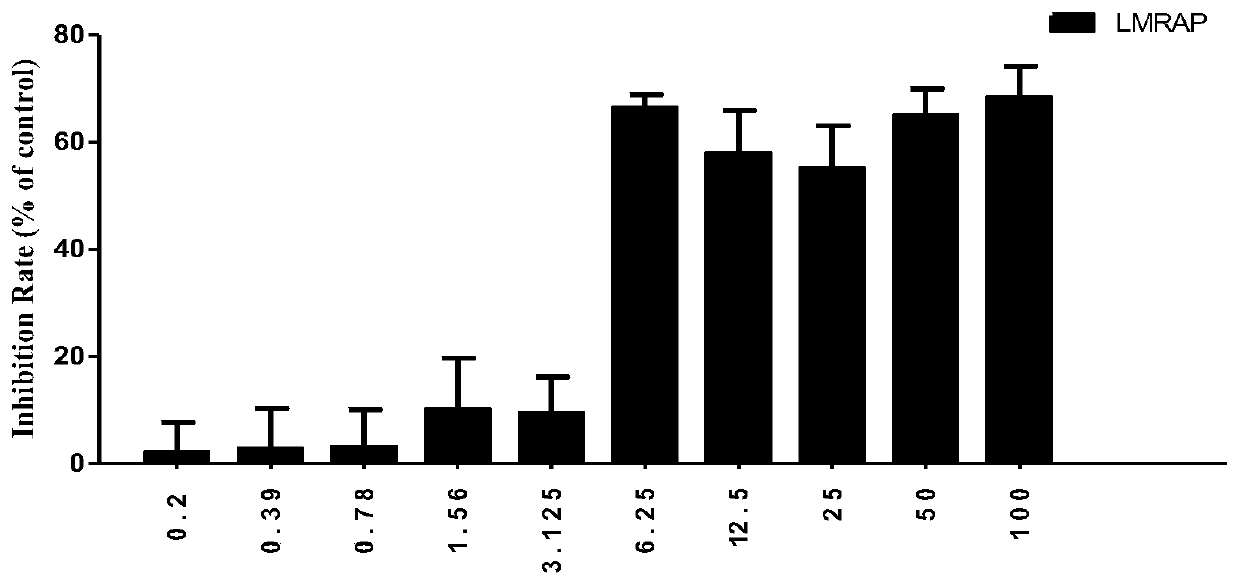
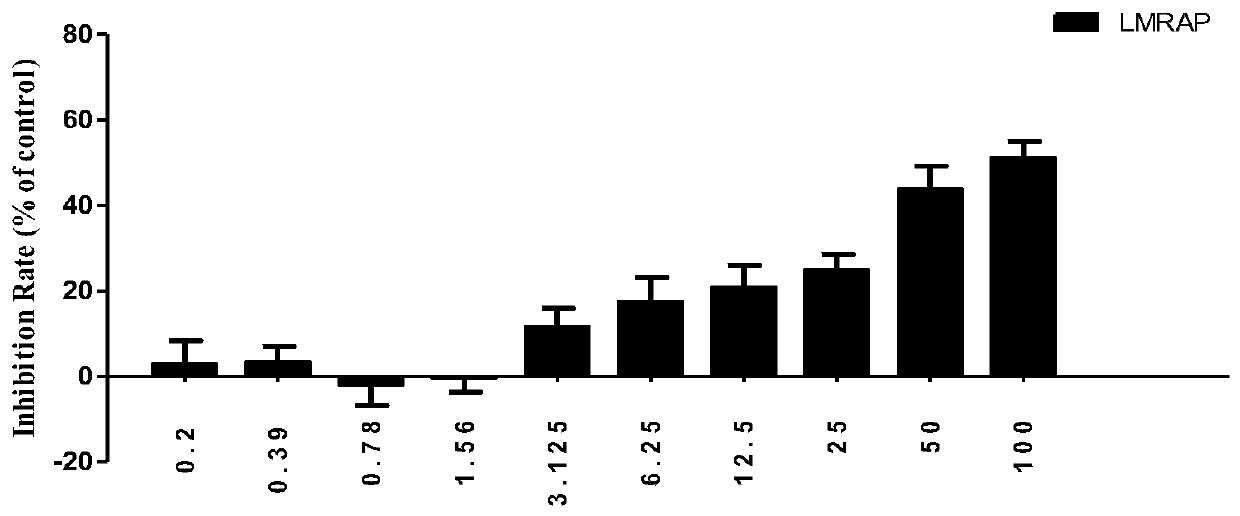
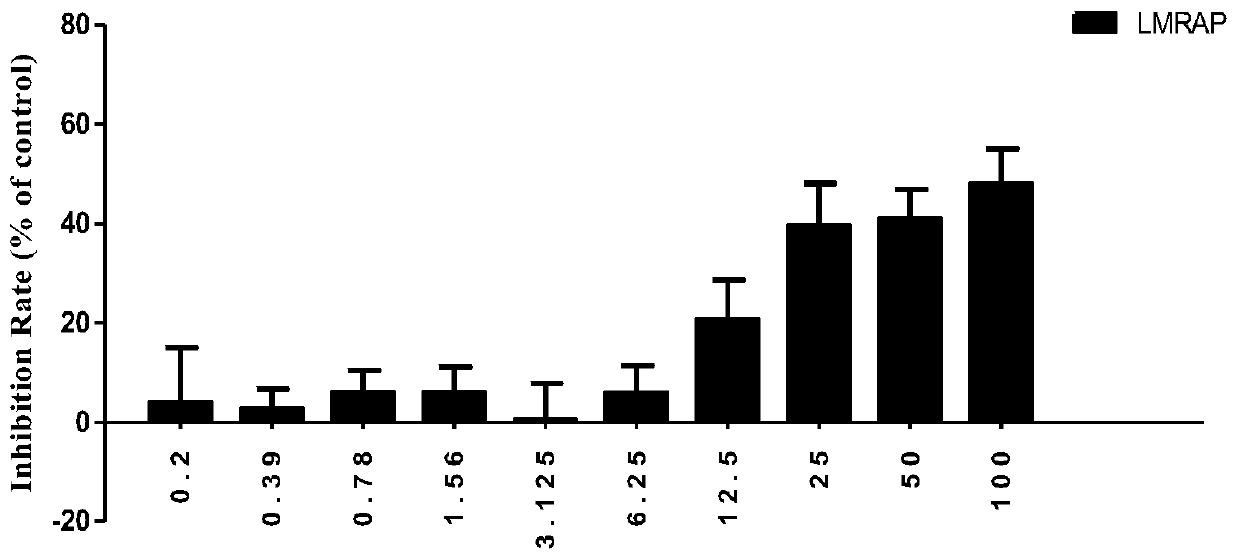
[0109] Inhibitory effect of LMRAP on the proliferation of various tumor cells
[0110] The CCK-8 method was used to detect the inhibitory effect of LMRAP obtained in Example 1 on the proliferation of various tumor cells, including prostate cancer cell 22RV1, gastric cancer cell MGC-803, ovarian cancer cell SKOV3, lung cancer cell A549, breast cancer cell MDA - MB-231, liver cancer cell SMMC-7721, colon cancer cell HCT-116, glioma cell U87, melanoma cell B16F10.
[0111] Cells in logarithmic growth phase were used for experiments. Cells were digested, counted, and made into 1×10 5 cell suspension per ml, inoculated in 96-well plate (100 μl / well), placed at 37°C, 5% CO 2 Cultivate in the incubator for 24 hours; add fusion protein LMRAP containing the corresponding concentration to each well, and set up a blank control group at the same time, with 6 multiple wells in each group; place the plate in the incubator for 72 hours, observe the cell morphology of each group under the m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com