Method for determining content of glucose in compound electrolyte glucose injection
A technology of glucose injection and compound electrolyte, which is applied in the directions of measuring devices, instruments, scientific instruments, etc., can solve problems such as undocumented, and achieve the effects of improving quality control, realizing separation, and simple and feasible determination methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
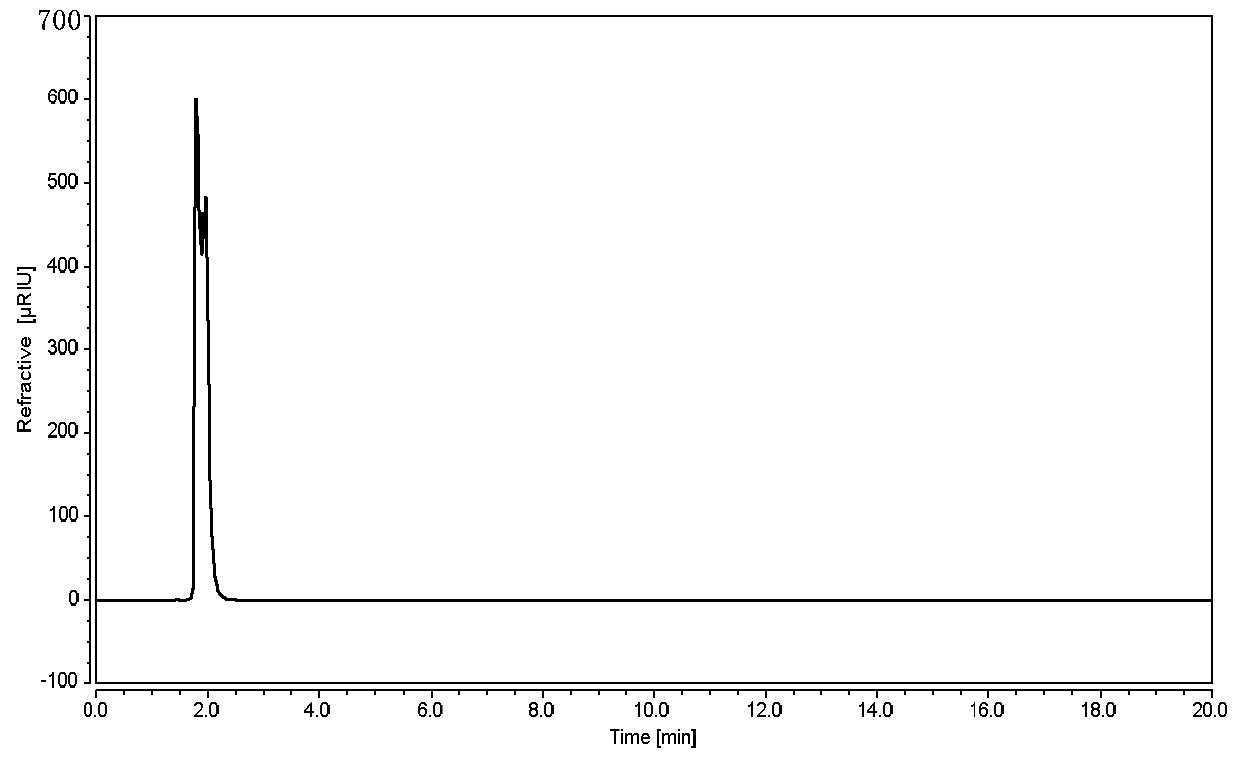
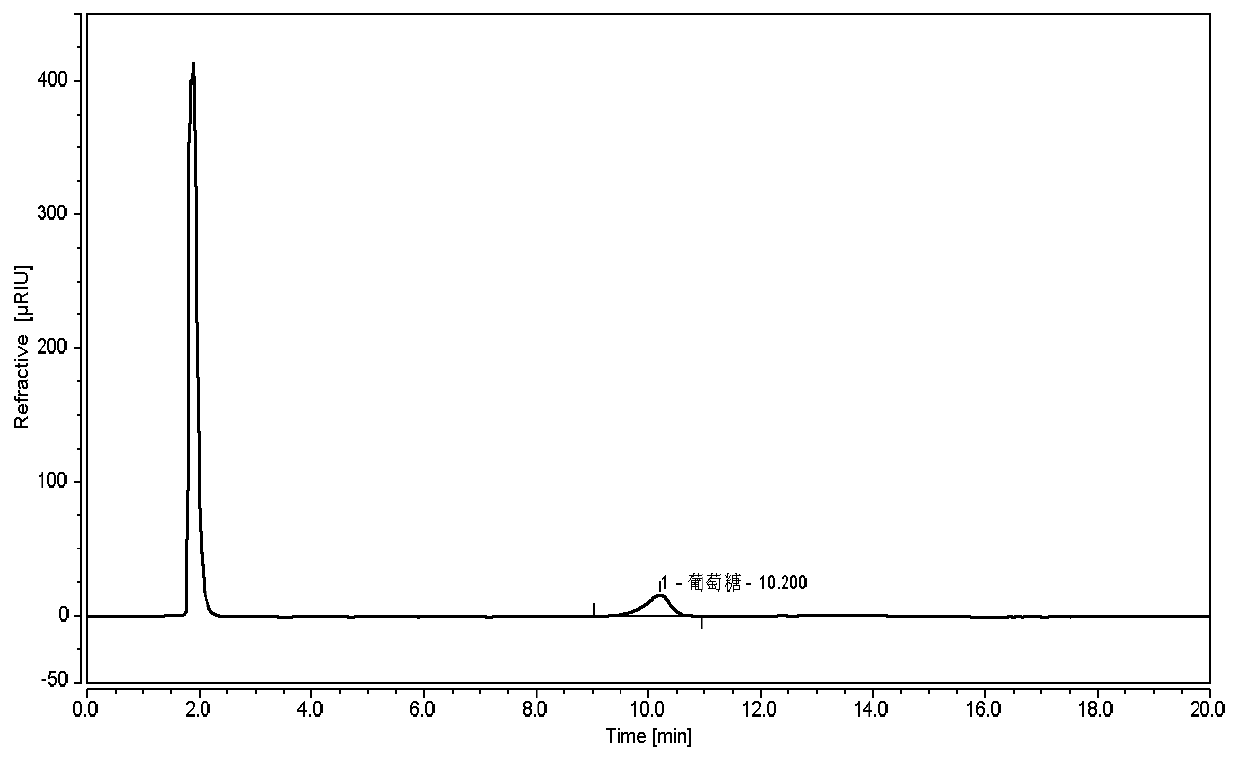
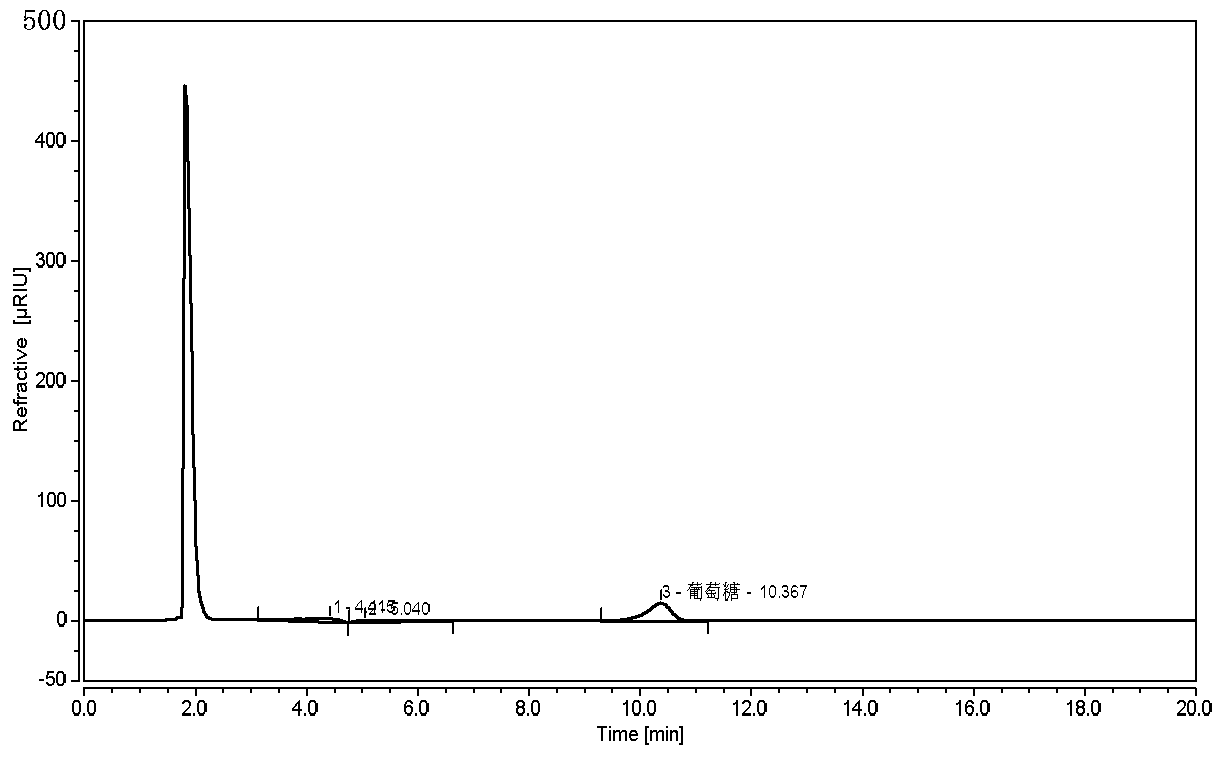
[0035] 1. Instruments and reagents
[0036] Thermo-Ultimate 3000 high performance liquid chromatograph (with differential refractive index detector), the chromatographic column is ShodexAsahipak NH2P-50 4E.
[0037] Glucose reference substance (batch number: R035J0, USP standard substance);
[0038] Glucose (batch number: 201711070, CSPC Shengxue Glucose Co., Ltd.);
[0039] Sodium chloride (batch number: 20170229, Jiangsu Province Qinfen Pharmaceutical);
[0040] Potassium chloride (batch number: 20170713-1, Jiangsu Province Qinfen Pharmaceutical);
[0041] Sodium lactate (batch number: 180102, Fuyue Industrial Co., Ltd.);
[0042] Compound electrolyte glucose MG3 injection (batch number: 8J74F11, China Otsuka Pharmaceutical Co., Ltd.);
[0043] Acetonitrile (Lot: 191994, Thermo Fisher)
[0044] 2. Method
[0045] 2.1 Preparation of reference solution
[0046] Accurately weigh 100mg of glucose reference substance, put it in a 10ml measuring bottle, add ultrapure water ...
Embodiment 2
[0078] 1. Instruments and reagents
[0079] Thermo-Ultimate 3000 high performance liquid chromatograph (with differential refractive index detector), the chromatographic column is ShodexAsahipak NH2P-50 4E.
[0080] Glucose reference substance (batch number: R035J0, USP standard substance);
[0081] Glucose (batch number: 201711070, CSPC Shengxue Glucose Co., Ltd.);
[0082] Sodium chloride (batch number: 20170229, Jiangsu Province Qinfen Pharmaceutical);
[0083] Potassium chloride (batch number: 20170713-1, Jiangsu Province Qinfen Pharmaceutical);
[0084] Sodium lactate (batch number: 180102, Fuyue Industrial Co., Ltd.);
[0085] Calcium chloride (batch number: 03170509, Tianjin Haiguang Pharmaceutical);
[0086] Compound sodium lactate glucose injection (batch number: 9D72F1, China Otsuka Pharmaceutical Co., Ltd.)
[0087] Acetonitrile (Lot: 191994, Thermo Fisher)
[0088] 2. Method
[0089] 2.1 Preparation of reference solution
[0090] Accurately weigh 100mg of g...
Embodiment 3
[0107] Durability test: Take the reference substance solution and adjust it according to the chromatographic conditions under "2.4", respectively flow rate 1.4ml / min, 1.6ml / min; column temperature 28°C, 32°C; test on the machine, see the results Figure 7-10 , within the adjusted range of chromatographic conditions, the tailing factor of the glucose chromatographic peak in the reference solution is not greater than 2.0, which is applicable to the detection of glucose.
[0108] 4. Discussion
[0109] 4.1 The content of glucose in the compound electrolyte glucose MG3 injection was determined by optical polarimetry. However, in practical applications, it is found that compound preparations often contain a variety of compounds with optical activity, and the traditional optical rotation method is not suitable for the determination of glucose content in compound preparations. In order to improve the quality standard of the product, an accurate, convenient and stable high-performanc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com