Targeted chimeric proteins and uses thereof
A chimeric protein, targeting moiety technology, applied in the direction of immunoglobulin, animal/human protein, anti-animal/human immunoglobulin, etc., can solve the problem of inability to treat
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0509] Example 1. VHH against murine SIRP1α binds SIRP1α and neutralizes the SIRP1α / CD47 interaction.
[0510] HEK293T cells were transiently transfected with the mouse SIRP1α expression plasmid and maintained at 37°C for 48 hours in DMEM medium supplemented with 10% FBS. The cells were separated, washed with PBS, and treated with the indicated concentration of purified His-tagged VHH in PBS supplemented with 1% FBS for 1 hour. The samples were washed with PBS and incubated with Alexa488-conjugated anti-His antibody (RnD system) in PBS supplemented with 1% FBS for 1 hour. The samples were measured on a FACSCalibur analyzer (BD Biosciences).
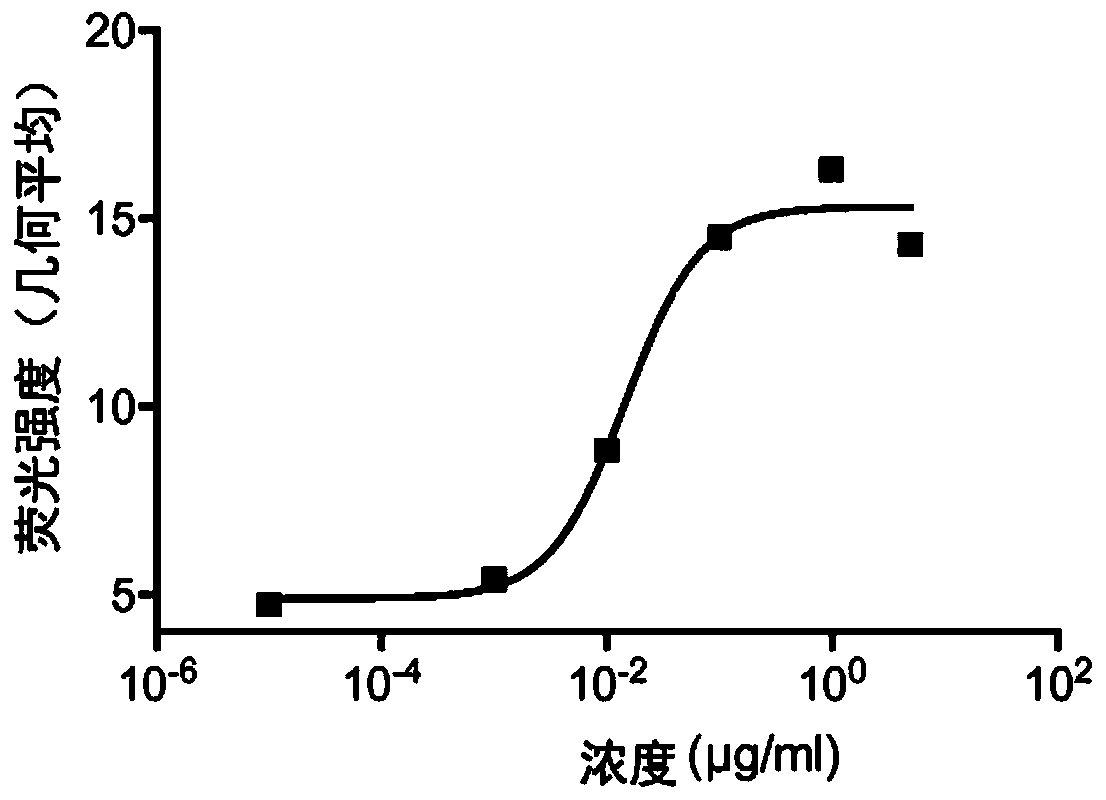
[0511] Such as Figure 1A As shown, a serial dilution of anti-mouse SIRP1αVHH was tested on cells expressing murine SIRP1α in a FACS-based mSIRPA binding assay. Plot the geometric mean of the fluorescence intensity. Anti-mouse SIRP1αVHH specifically binds to mouse SIRP1α ( Figure 1B ).
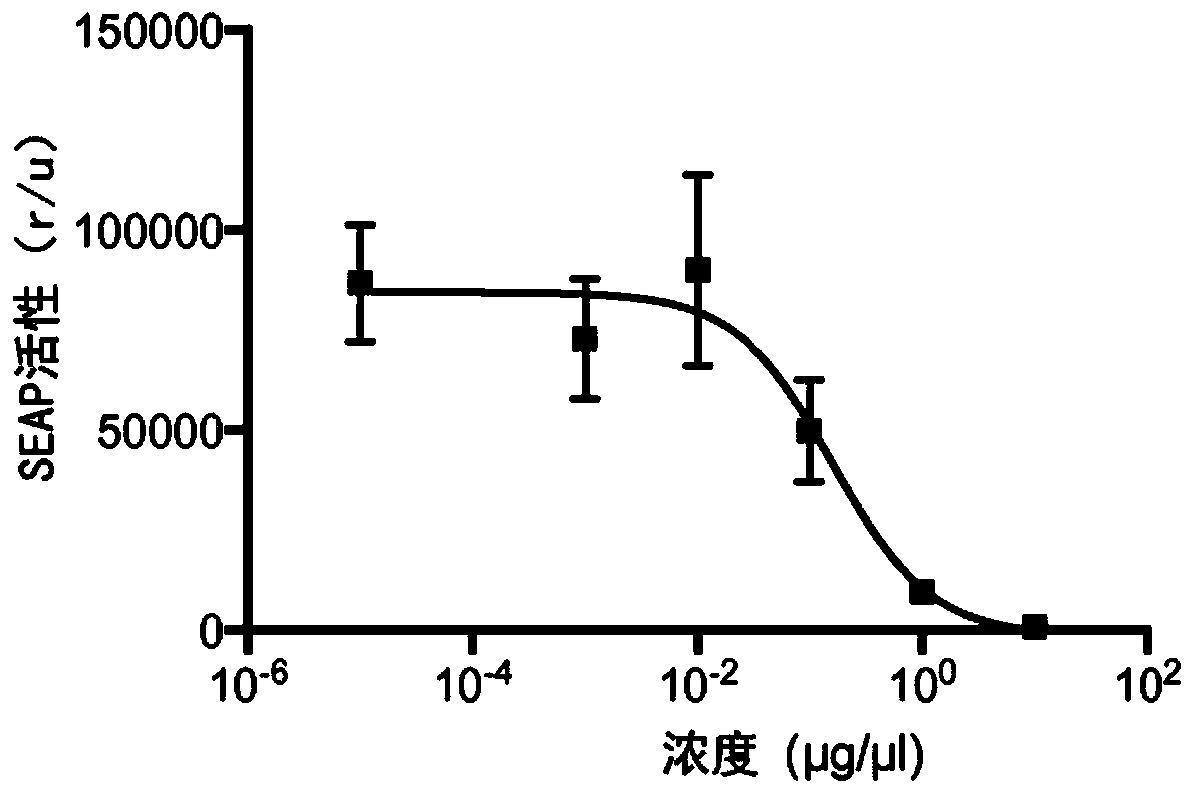
[0512] It was also checked whether the anti-mouse SIR...
Embodiment 2
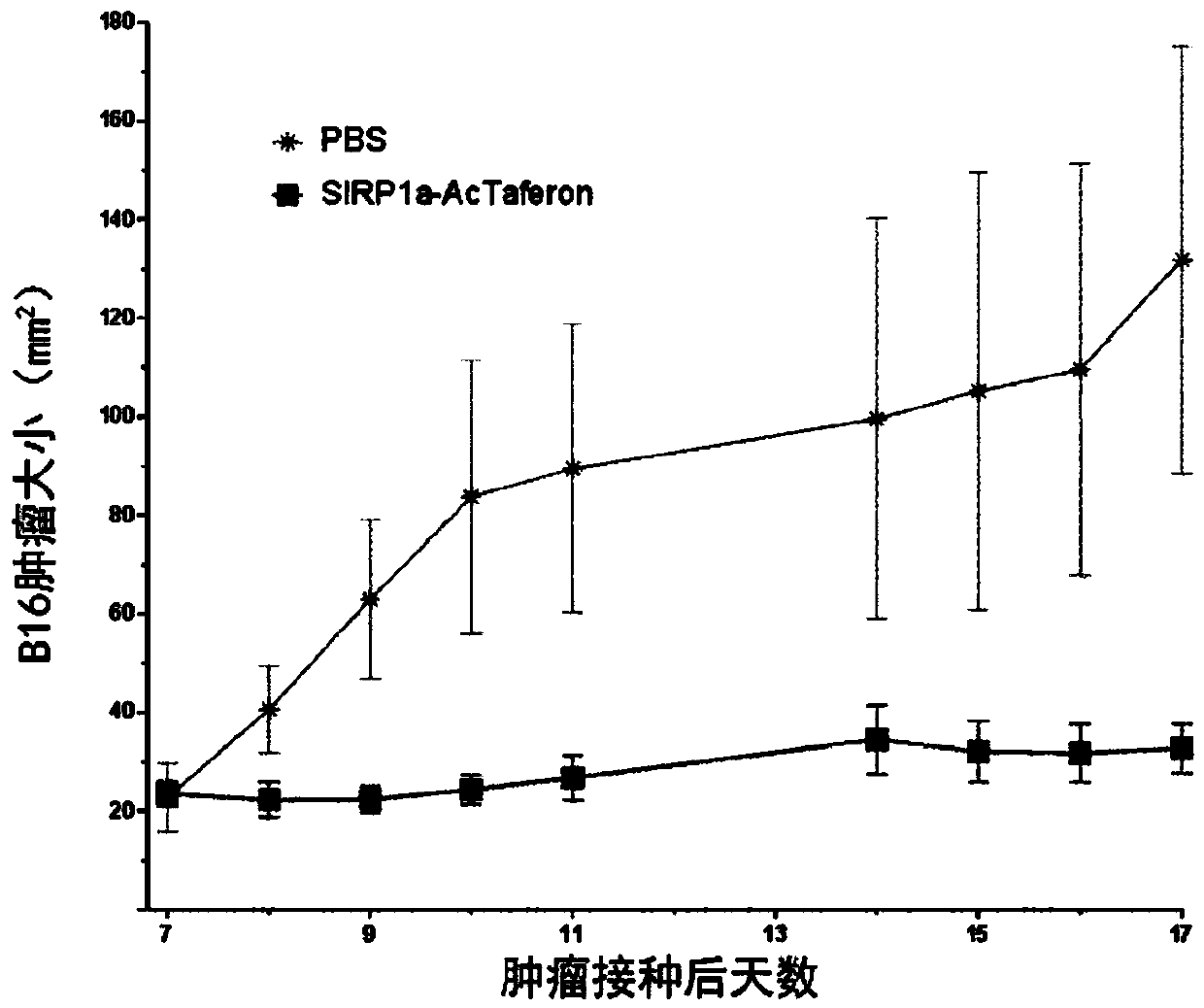
[0515] Example 2. Anti-tumor effect of SIRP1α bispecific chimera in vivo
[0516] In the B16 model, the anti-mouse Sirp1αVHH / human IFN Q124R chimera was used for in vivo studies in mice.
[0517] The chimera targeting SIRP1α (hIFNα2Q124R is coupled to the N-terminal neutralization VHH specific to mouse SIRP1α via a 20xGGS-linker) was constructed in pHen6 vector, and the His-tagged SIRP1a-chimera was constructed in E. coli Mass production. Cultivate the bacteria until the stationary phase (OD600 is 0.7-0.8), and then add IPTG (Bioscientific) to activate the LacZ promoter. After culturing overnight, the cell supernatant was collected. The protein in the periplasmic fraction was released by osmotic shock using a sucrose solution, and purified by immobilized metal ion chromatography (IMAC) on HiTrap Sepharose resin loaded with Kobalt ions (Clontech, Takara Biotechnology). After binding the protein, the column was washed with 0.5% EMPIGEN (Calbiochem, Millipore), 0.5% CHAPS (Sigma-A...
Embodiment 3
[0521] Example 3. SIRP1α bispecific chimera
[0522] The anti-mouse Sirp1αVHH / anti-mouse PD-L1 VHH / human IFN Q124R bispecific chimera was studied. Specifically, FACS analysis was performed to quantify STAT1 phosphorylation in the mouse PD-L1 positive B16 cell line.
[0523] B16 cells were stimulated with Sirp1α bispecific chimera in DMEM medium supplemented with 10% FBS at 37°C for 15 minutes. After stimulation, the cells were fixed by adding 1 volume of Fix Buffer I (BD Biosciences) at 37°C for 10 minutes, and permeabilized by resuspending in 2 volumes of Perm III Buffer I (BD Biosciences) on ice for 30 minutes. The samples were stained with anti-STAT1 pY701 antibody (BD Biosciences) at 4°C for 20 minutes, and analyzed with FACSCalibur (BD Biosciences) and CellQuest Pro version 4.0.2 software (BD Biosciences).
[0524] Such as image 3 As shown, B16 cells were stimulated with 100ng / ml anti-mouse Sirp1αVHH / anti-mouse PD-L1VHH / human IFNQ124R bispecific chimera and Bcll10 VHH-human ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com