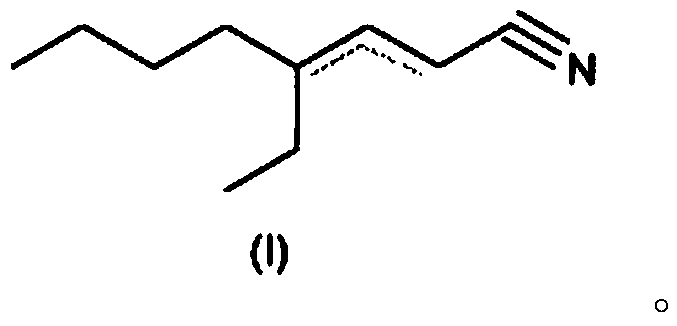
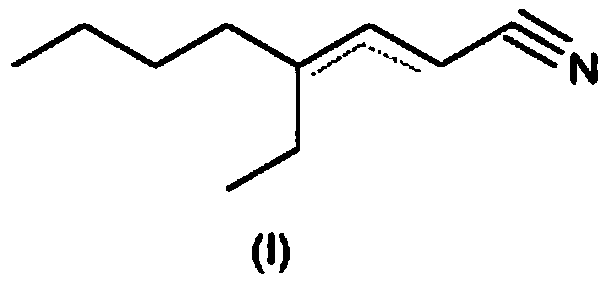
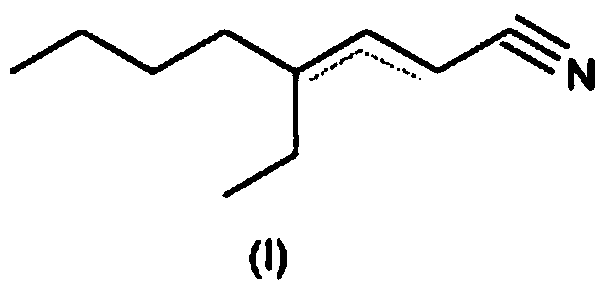
4-ethyl-octene-2/3-nitrile as a fragrance
A fragrance and fragrance technology, applied in the field of changing and/or strengthening specific fragrance notes, and can solve the problems of strong changes in sensory properties, affecting human tissue tolerance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0126] Example 1: Preparation of 4-ethyl-octene-2-carbonitrile / 4-ethyl-octene-3-carbonitrile
[0127] 254 g of 2-ethylhexanal, 38 g of ammonium acetate and 46 g of acetic acid were supplied to 520 g of toluene in a 1000 ml stirrer with a water separator. Subsequently, 189 g of cyanoacetic acid were added in parts at room temperature while stirring. Thereafter, it was refluxed in a water separator for 14 hours and washed with 10% sulfuric acid, 5% sodium hydroxide or water, respectively, after cooling. The organic phase was confined and the crude product (280 g) was fractionated under vacuum on a 40 cm Vigreux column.
[0128] Yield: 190g (63.9% of theoretical value), boiling point: 80°C to 85°C / 0.8mbar GC evaluation (20m DB-WAX, inner diameter 0.18μm / 60-9-220°C temperature programmed evaporator) product from 4 One isomer composition: 4.5% (E / Z) 4-ethyl-octene-2-carbonitrile and 95.5% (E / Z) 4-ethyl-octene-3-carbonitrile.
[0129] (E / Z)4-Ethyl-octene-2-carbonitrile
[0130]...
Embodiment 1a
[0135] Example 1a: Preparation of 4-ethyl-octene-2-carbonitrile / 4-ethyl-octene-3-carbonitrile
[0136]254 g of 2-ethylhexanal and 189 g of cyanoacetic acid were introduced into 500 ml of cyclohexane in a 1000 ml stirrer with water separator and dropping funnel. Thereafter, 65 g of 3-picoline were added under stirring and boiling conditions. Subsequently, it was refluxed in a water trap for 26 hours and washed with 10% sulfuric acid, 5% sodium hydroxide or water, respectively, after cooling. The organic phase was bound and the crude product (250 g) was obtained. The crude product consisted of 4 isomers: 16.5% (E / Z) 4-ethyl-octene-2-carbonitrile and 63.9% (E / Z) 4-ethyl-octene-3-carbonitrile.
[0137] The crude product was fractionated on a canned-column in vacuo.
[0138] Product yield: 37.5 g (E / Z) 4-ethyl-octene-2-carbonitrile (95.4%)
[0139] Boiling point: 75°C-80°C / 0.8 mbar
[0140] GC analysis (20m DB-WAX, inner diameter 0.18μm / 60-9-220℃ programmed temperature evapo...
example 2
[0141] Example 2: Perfume Composition (Fragrance Composition)
[0142]
[0143]
[0144] According to the perfumer's opinion, the perfume composition becomes drier, powdery, smooth transition by adding 0.5% by weight of a compound of formula (Ia) or (Ib) or a mixture consisting of (Ia) or (Ib) Harmonious, where pronounced iris violet and rose notes are added and intensified woody and floral aspects. Compositions or uses according to the invention give the compositions individuality and combine different odor elements. Example 3: Perfume Composition (Fragrance Composition)
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com