Insulin-like growth factor binding protein 4 mutant and pharmaceutical application thereof
A growth factor, insulin-like technology, applied in the fields of bioengineering and biomedicine, can solve the problems of high cost, toxic and side effects, poor effect and prognosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
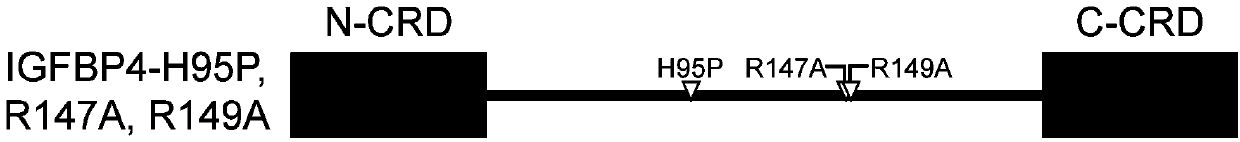
[0108] Embodiment 1, IGFBP4-H95P, R147A, the preparation of R149A protein
[0109] 1. Construction of recombinant vectors expressing IGFBP4-H95P, R147A, R149A mutants
[0110] Human mRNA extracted by conventional methods was reverse-transcribed to obtain cDNA. Using the cDNA as a template, use the primer pair 1 corresponding to both sides of the ORF frame of the IGFBP-4 gene (SEQ ID NO: 1), obtain the cDNA sequence by reverse transcription, and perform PCR amplification using DNA polymerase: obtain human IGFBP-4 amplification product. Detected by 1% agarose gel electrophoresis, the length of the amplified product was consistent with the predicted value.
[0111] The obtained coding nucleotide sequence of human IGFBP-4 was cloned into pcDNA3.1V5-6HIS vector (purchased from Agilent Technologies Genomics) to obtain the recombinant vector pcDNA3.1V5-6HIS / IGFBP4. This recombinant vector can be used to transfect HEK293 cells to express wild-type IGFBP-4 protein by the same proced...
Embodiment 2
[0132] Example 2, IGFBP4-H95P, R147A, R149A protein does not bind IGF I or IGF II (ie IGFs)
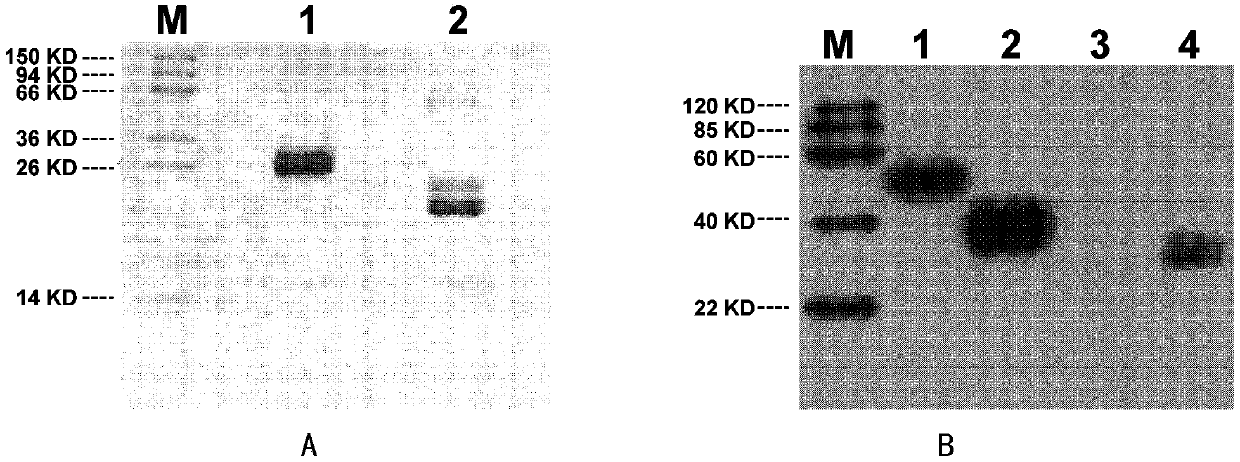
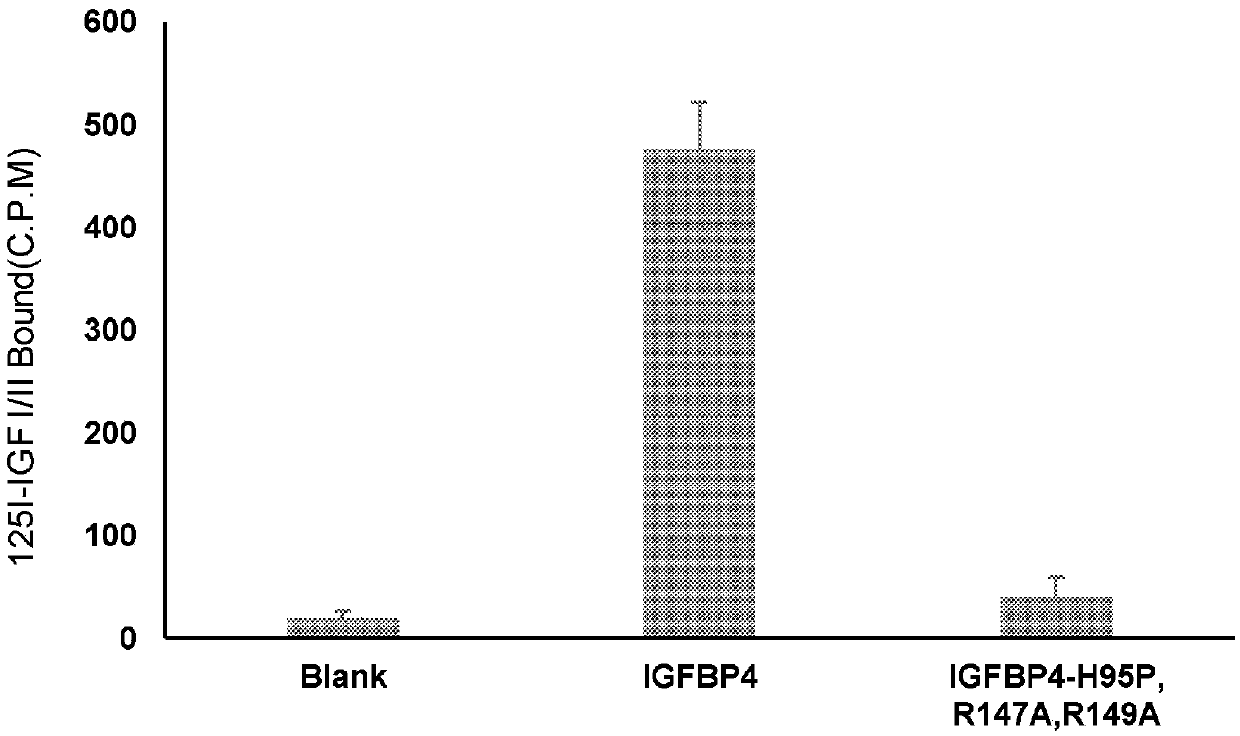
[0133] Obtain wild IGFBP4 protein and mutated IGFBP4-H95P as in Example 1, R147A, R149A protein, and SDS-PAGE (100mM Tris, pH 6.8, 10% SDS, 0.01% phenol blue) loading buffer containing β-mercaptoethanol Mix and separate by 10% SDS-PAGE gel. The gel protein was transferred to a nitrocellulose membrane for conventional 125I-IGF-I and 125I-IGF-II (purchased from Amersham Pharmacia Biotech, Freibury, Germany) Western ligand blotting ( 125 I-IGF-I and 125 I-IGF-II Western ligand blotting analysis). The amount of natural IGFBP4 protein used and mutated IGFBP4-H95P, R147A, and R149A protein was 105ng each; 125 I-IGF-I and 125 The dosage of I-IGF-II was 250 μCi / μg protein. The analysis results are shown in image 3 . image 3 In , the IGF-binding activity of the protein was quantified by gamma counting the analyzed protein bands, and the background radioactivity of the nitrocellulose m...
Embodiment 3
[0134] Example 3, IGFBP4-H95P, R147A, R149A proteins do not bind to IGF II
[0135] IGF II is known to stimulate DNA synthesis in human osteosarcoma cell MG63, and this stimulation can be neutralized by the binding protein IGFBP4 of IGFs.
[0136] Human osteosarcoma MG63 cells (purchased from American Type Culture Collection; Rockville, MD; CRL 1427) were cultured in vitro until the cells were basically confluent, and fresh DMEM medium (supplemented with 0.1% calf serum) was replaced for 20 hours. , adding natural IGFBP4 and IGFBP4-H95P, R147A, R149A proteins as obtained in Example 1 at different concentrations to the same volume of medium for culturing the same number of cells, respectively. BSA at the same concentration was used as a control. Under normal cell culture conditions, incubate for 48h. Collect the cultured cells, use the CytQUANT cell proliferation kit (purchased from Life Technologies, Beijing), mix the collected cells with lysis buffer and dyeing solution acc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com