Catalyst for co-production of low carbon alkenes from carbon dioxide hydrogenation to gasoline and preparation method of catalyst
A technology for carbon dioxide and low-carbon olefins, applied in the field of catalysts and their preparation, can solve the problems of low CO2 conversion rate, high carbon monoxide selectivity, low product yield and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
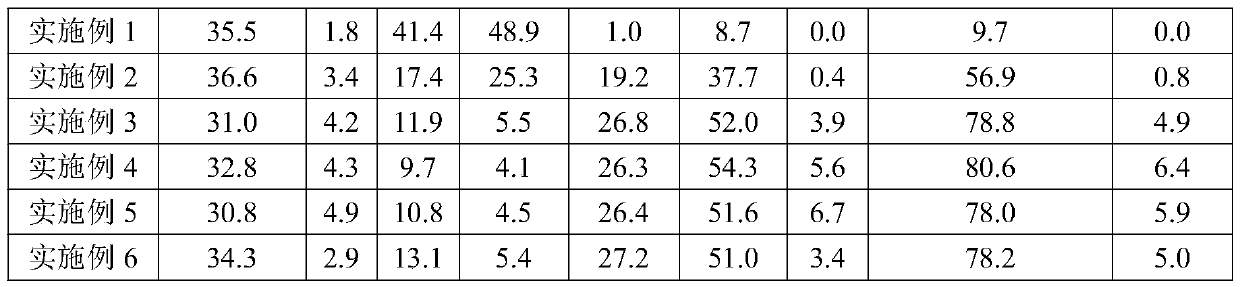
Examples
Embodiment 1
[0024] Example 1: Preparation of 8.3wt.% Fe1.7wt.% Co / γ-Al 2 o 3 catalyst
[0025] Weigh 4.42mmolFe(NO 3 ) 3 9H 2 O and 0.93mmolCo(NO 3 ) 2 ·6H 2 O was added to 3g of deionized water, ultrasonicated to form a homogeneous solution, and then 3g of γ-Al was weighed 2 o 3 (Carrier) was added to the above solution and kept stirring to make γ-Al 2 o 3 Mix well with the complexing solution, put the stirred solution in an oven and dry overnight at 80°C, then take out the solid, grind the solid with an agate mortar, and finally put the ground solid in a muffle furnace Calcined at 500°C for 4h, the catalyst obtained at this time is recorded as 8.3wt.%Fe1.7wt.%Co / γ-Al 2 o 3 . The contents of Fe and Co are based on the carrier (γ-Al 2 o 3 ) weight calculation.
Embodiment 2
[0026] Embodiment 2: Prepare 8.3wt.% Fe1.7%wt.Co1.8wt.%Na / γ-Al 2 o 3 Catalyst weighs 4.42mmolFe (NO 3 ) 3 9H 2 O, 0.93mmolCo(NO 3 ) 2 ·6H 2 O and 1.21 mmol EDTA2Na 2H 2 O was added to 4 g of deionized water, and ultrasonically formed a uniform complexation solution without precipitation under heating conditions at 50 °C, and then weighed 3 g of γ-Al 2 o 3 (Carrier) is added to the above complex solution and stirred continuously to make γ-Al 2 o 3 Mix well with the complexing solution, put the stirred solution in an oven and dry overnight at 80°C, then take out the solid, grind the solid with an agate mortar, and finally put the ground solid in a muffle furnace Calcined at 500°C for 4h, the catalyst obtained at this time was recorded as 8.3wt.%Fe1.7wt.%Co1.8wt.%Na / γ-Al 2 o 3 . The contents of Fe, Co and Na are all determined according to the carrier (γ-Al 2 o 3 ) weight calculation, the same below.
Embodiment 3
[0027] Example 3: Preparation of 8.3wt.% Fe1.7wt.% Co3.7wt.% Na / γ-Al 2 o 3 catalyst
[0028] Weigh 4.42mmolFe(NO 3 ) 3 9H 2 O, 0.93mmolCo(NO 3 ) 2 ·6H 2 O and 2.41 mmol EDTA2Na 2H 2 O was added to 4 g of deionized water, and ultrasonically formed a uniform complexation solution without precipitation under heating conditions at 50 °C, and then weighed 3 g of γ-Al 2 o 3 (Carrier) is added to the above complex solution and stirred continuously to make γ-Al 2 o 3 Mix well with the complexing solution, put the stirred solution in an oven and dry overnight at 80°C, then take out the solid, grind the solid with an agate mortar, and finally put the ground solid in a muffle furnace Calcined at 500°C for 4h, the catalyst obtained at this time was recorded as 8.3wt.%Fe1.7wt.%Co3.7wt.%Na / γ-Al 2 o 3 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com