Method for inducing human skin fibroblasts to differentiate into adipocytes in vitro
A fibroblast, inducing differentiation technology, applied in biochemical equipment and methods, animal cells, vertebrate cells, etc., can solve the problem of inability to induce differentiation of human skin fibroblasts, inability to apply skin wound repair and scar repair, tissue source Restriction and other problems to achieve the effect of promoting differentiation into adipocytes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Embodiment 1 The method for inducing human skin fibroblasts to differentiate into adipocytes in vitro
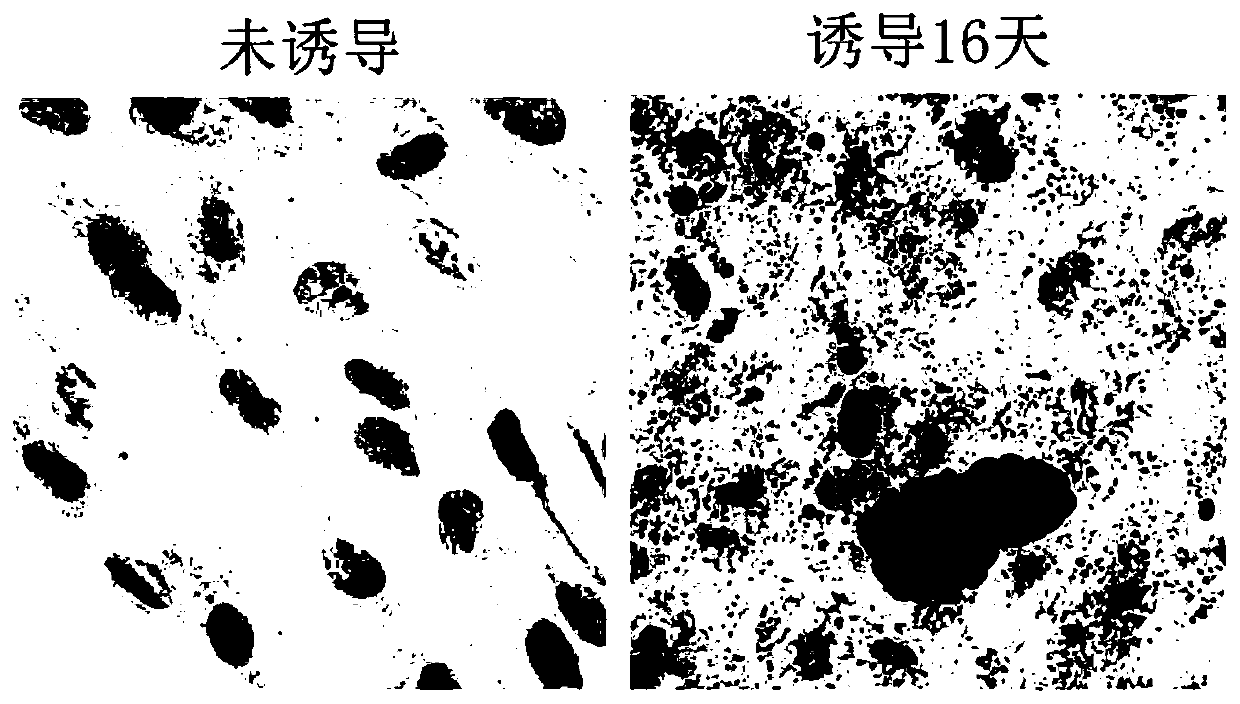
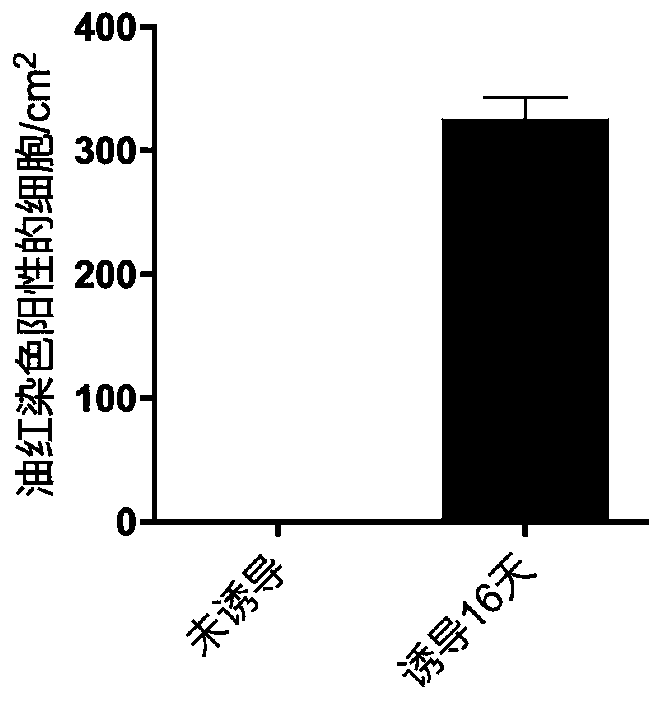
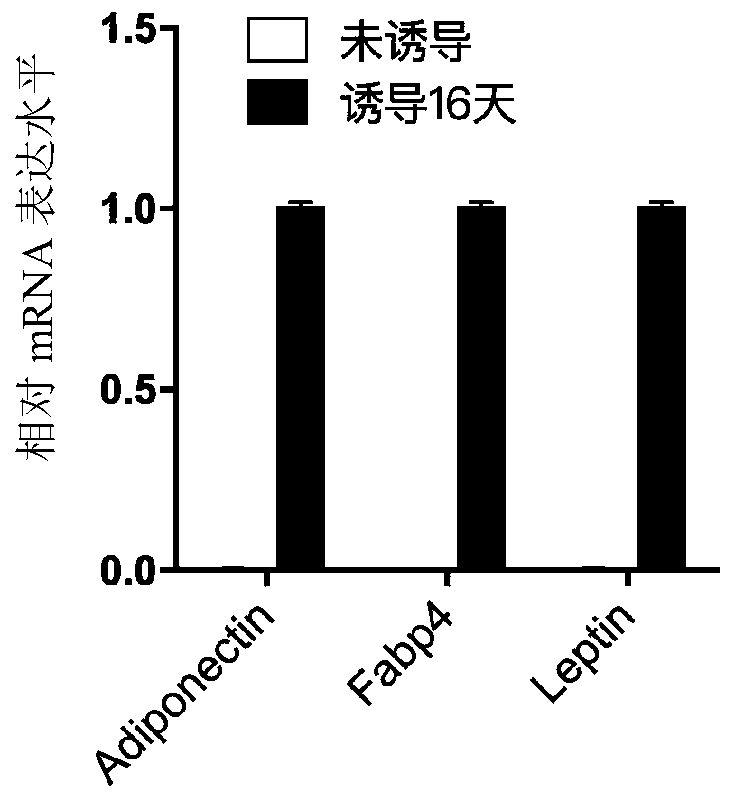
[0045] In this example, the isolated primary human skin fibroblasts (passage 6) were taken as an example, and the differentiation mode of "6+2+8=16 days" was adopted to induce the differentiation of primary human skin fibroblasts into adipocytes in vitro . The specific method is as follows:
[0046] 1. Make about 10 5 HDF cells were subcultured into a 35mm culture dish, added with 4ml of basal medium, and cultured in an incubator for 2 days. Cell culture conditions are 37 °C, 5% CO 2 , the same below.
[0047] 2. When the confluence of the cells reaches 95%, replace the medium with 3ml induction differentiation medium ①, replace it every two days, and induce culture for 6 days.
[0048] 3. Replace the induction differentiation medium ① with 3ml induction differentiation medium ②, and culture for 2 days.
[0049] 4. Replace the differentiation-inducing medium ② wit...
Embodiment 2
[0086] Example 2 Induced Differentiation Medium ① Composition Optimization Experiment
[0087] The specific experimental steps of this example are the same as in Example 1, and the differentiation mode of "6+2+8=16 days" is also adopted to induce differentiation of primary human skin fibroblasts (6th passage). The difference is that the following adjustments were made to the composition of the induction differentiation medium ①:
[0088] Control group: differentiation induction medium ① containing 1 μM dexamethasone, 20 μg / mL insulin, 2 μM rosiglitazone, 0.5 mM 1-methyl-3-isobutylxanthine;
[0089] Dexamethasone-free group: differentiation induction medium ① containing 20 μg / mL insulin, 2 μM rosiglitazone, 0.5 mM 1-methyl-3-isobutylxanthine;
[0090] Insulin-free group: induction differentiation medium ① containing 1 μM dexamethasone, 2 μM rosiglitazone, 0.5 mM 1-methyl-3-isobutylxanthine;
[0091] No rosiglitazone group: differentiation induction medium ① containing 1 μM de...
Embodiment 3
[0097] Embodiment 3 The influence of generation number of human skin fibroblasts on the effect of inducing differentiation
[0098] The specific experimental steps of this example are the same as in Example 1, and the differentiation mode of "6+2+8=16 days" is also adopted to induce differentiation of primary human skin fibroblasts of different passages. The 3rd, 7th, 10th and 12th passage cells were selected for experiments.
[0099] The results of oil red staining are shown in Figure 7 , as shown in the figure, with the increase of the cell generation, the oil red staining gradually decreased, and the oil red staining was almost undetectable in the 12th passage cells.
[0100] For statistical results of oil red staining, see Figure 8 , as shown in the figure, with the increase of cell generation, the number of oil red stained cells gradually decreased, and the number of oil red stained cells in the 12th generation cells was almost zero.
[0101] For the test results of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com