Mesenchymal stem cell, and preparation method and application thereof
A technology of stem cells and embryoid bodies, applied in the field of stem cell biology, can solve the problems of large demand for clinical cells, unstable MSC effect, and limited number of MSCs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] A method for preparing mesenchymal stem cells, comprising the steps of:
[0088] S1: Human pluripotent stem cells form embryoid bodies;
[0089] S2: Differentiation of embryoid bodies to mesoderm cells;
[0090] S3: Differentiation of mesoderm cells into mesenchymal stem cells.
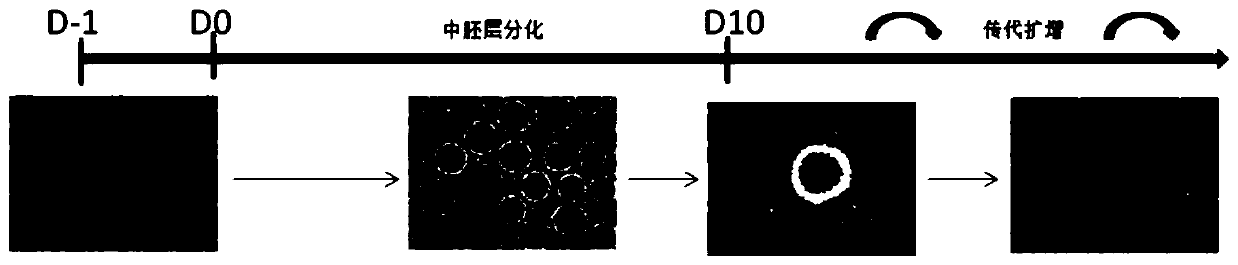
[0091] Specifically, the flow chart is as figure 1 As shown, the steps are as follows:
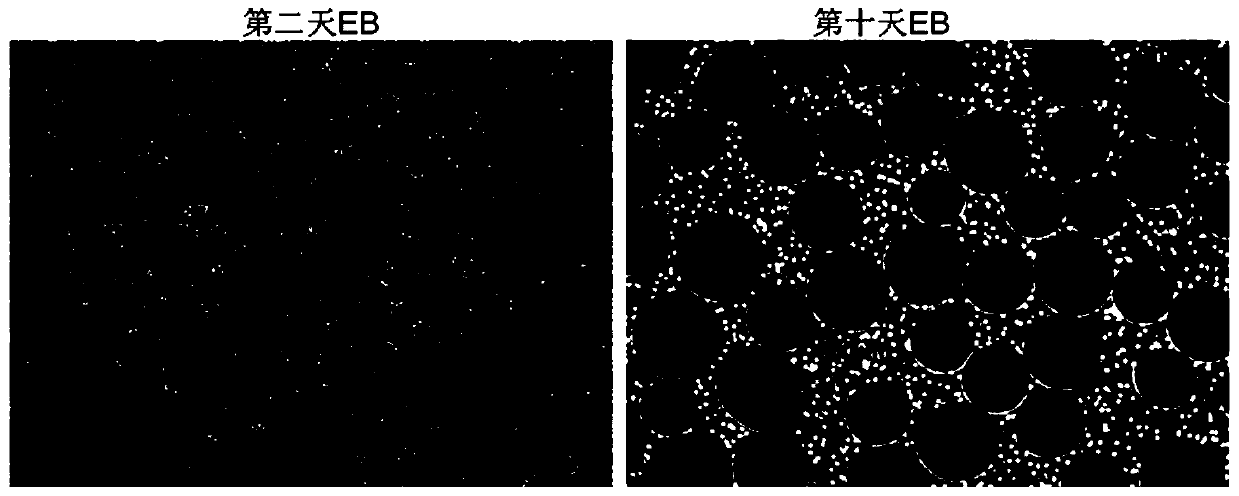
[0092] (1) Human pluripotent stem cells form embryoid bodies (D-1~D0)
[0093] The hPSCs used in the experiment have been strictly verified for pluripotency (expressing various pluripotency markers, and can form teratomas including inner, middle and outer germ layers in immunodeficient mice). hPSCs are normally cultured in hPSC maintenance medium, the medium used is E8 or TeSR or other similar medium.
[0094] In this example, human pluripotent stem cells were prepared by the method disclosed in patent CN 108085299A.
[0095] After human pluripotent stem cells were cultured to a confluence of 70-90%,...
Embodiment 2
[0114] This example studies the effects of 2D culture and 3D culture on the number of iMSCs obtained. The only difference between the 2D culture in this example and Example 1 is that 2D culture is used in the whole induction process.
[0115] The experimental results are shown in Table 3:
[0116] table 3
[0117]
[0118] It can be concluded from Table 3 that with the 3D differentiation method, the cells can grow out of the EBs and get iMSCs with a cell volume about 10 to 15 times that of the initial cells. Twice as many cells were obtained and 1 / 4 the amount of medium was used. Therefore, 3D culture is preferred for large-scale production of iMSCs.
Embodiment 3
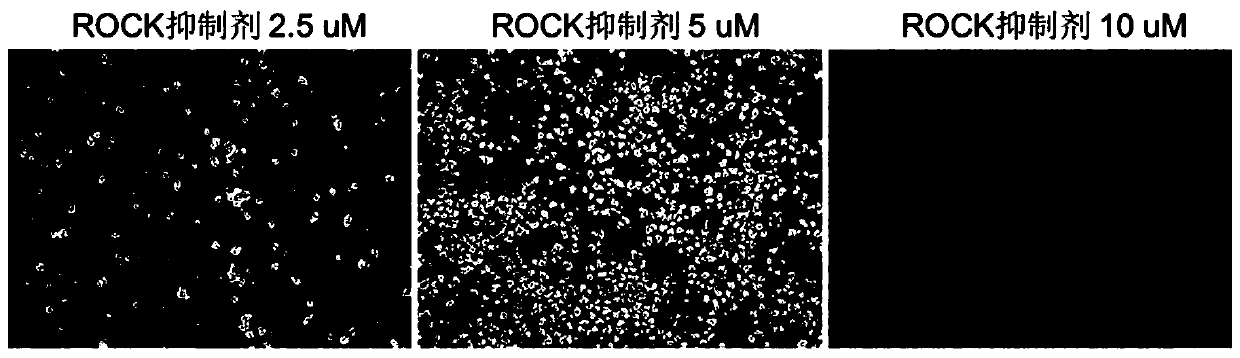
[0120] This example studies the influence of different Rock inhibitor concentrations on the experimental results. The ROCK inhibitor concentrations were set at 2.5, 5 and 10 μM, respectively. The result is as figure 2 As shown, when the concentration of ROCK inhibitor was 2.5 and 5 μM, the EBs formed by pluripotent stem cells were small and heterogeneous in shape; when the concentration of ROCK inhibitor was 10 μM, the EBs formed were very round and uniform in shape.
[0121]Thus, the concentration of ROCK inhibitor plays a large role in the formation of EBs during the first 8–32 hours of our differentiation method.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com