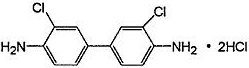
Method for synthesizing 3,3'-dichlorobenzidine hydrochloride through hydrochloric acid translocation
A technology of dichlorobenzidine hydrochloride and dichlorohydrin azobenzene is applied in the chemical field, and can solve the problems of slow reaction, viscous material, large mass transfer resistance, etc., and achieves easy operation, reduced viscosity, and improved reaction system. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 200 g of 2,2'-dichlorohydroazobenzene and 180 g of toluene into the stirred reaction equipment, start stirring, and add 1.2 g of emulsifier TX-4 and 0.3 g of sodium dodecylsulfonate after completely dissolving , and then slowly add 380 g of 31% hydrochloric acid dropwise, and drop it in 2 h. After the dropwise addition, the temperature was raised to 40-50°C for the transposition reaction. After about 4 hours, the content of 2,2'-dichlorohydroazobenzene in the solution was detected to be less than 0.5%, and the transposition reaction was completed. The total operation time was 6 hours. The transposition solution was dissolved in water, and the lower aqueous phase was separated, decolorized and filtered to obtain the finished product of 3,3'-dichlorobenzidine hydrochloride with a yield of 89.5%.
[0021] Note: In Example 1, the mass ratio of 2,2'-dichlorohydroazobenzene to toluene is 1:0.9, and the additives added are sodium dodecylsulfonate and emulsifier TX-4, and t...
Embodiment 2
[0023] Add 200 g of 2,2'-dichlorohydroazobenzene and 200 g of toluene into the stirred reaction equipment, start stirring, and add 1 g of emulsifier TX-7 and 0.2 g of dodecylbenzenesulfonic acid after completely dissolving Sodium, then slowly add 400 g of 31% hydrochloric acid dropwise, and drop it over 1.5 h. After the dropwise addition, the temperature was raised to 40-50°C for the transposition reaction. After about 4 hours, the content of 2,2'-dichlorohydroazobenzene in the solution was detected to be less than 0.5%, and the transposition reaction was completed. The total operation time was 5.5 hours. The transposition solution was dissolved in water, the lower aqueous phase was separated, decolorized and filtered to obtain the finished product of 3,3'-dichlorobenzidine hydrochloride with a yield of 88.7%.
[0024] Note: In Example 2, the mass ratio of 2,2'-dichlorohydroazobenzene to toluene is 1:1, and the additives added are sodium dodecylbenzenesulfonate and emulsifier ...
Embodiment 3
[0026] Add 200 g of 2,2'-dichlorohydroazobenzene and 220 g of toluene into the stirred reaction equipment, start stirring, add 1.6 g of emulsifier TX-9 after completely dissolving, then slowly add 420 g of TX-9 with a concentration of 31 % hydrochloric acid, drop over 2.5 h. After the dropwise addition, the temperature was raised to 40-50°C for transposition reaction. After about 3.5 hours, the content of 2,2'-dichlorohydroazobenzene in the solution was detected to be less than 0.5%, and the transposition reaction was completed. The total operation time was 6 hours. The transposition solution was dissolved in water, the lower aqueous phase was separated, decolorized and filtered to obtain the finished product of 3,3'-dichlorobenzidine hydrochloride with a yield of 89.7%.
[0027] Note: In Example 3, the mass ratio of 2,2'-dichlorohydroazobenzene to toluene is 1:1.1, the additive added is emulsifier TX-9, and the auxiliary agent and 2,2'-dichlorohydroazo The mass ratio of benz...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com