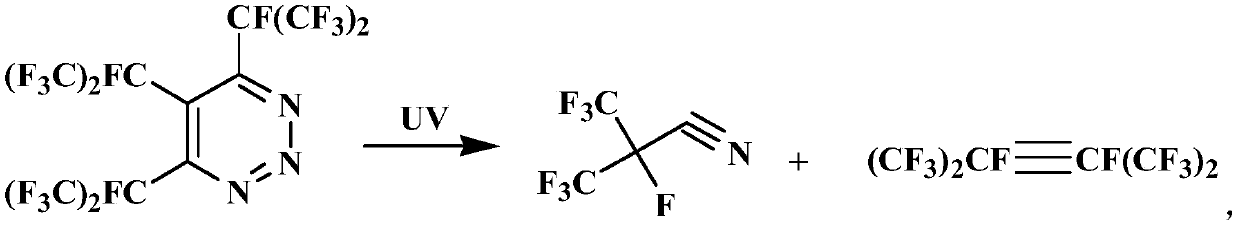
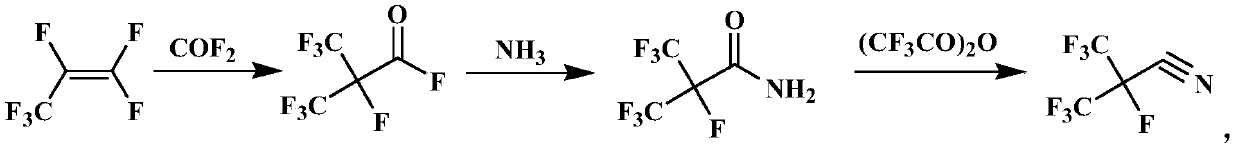
Preparation method for perfluoroisobutyronitrile
A technology of heptafluoroisobutyronitrile and heptafluoroisobutyramide is applied in the field of preparation of heptafluoroisobutyronitrile, can solve the problems of high cost, difficult to store in the system, low yield and the like, and achieves mild reaction conditions and no three wastes Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] The embodiment of the present application provides the first preparation method of heptafluoroisobutyronitrile, the specific steps are as follows:
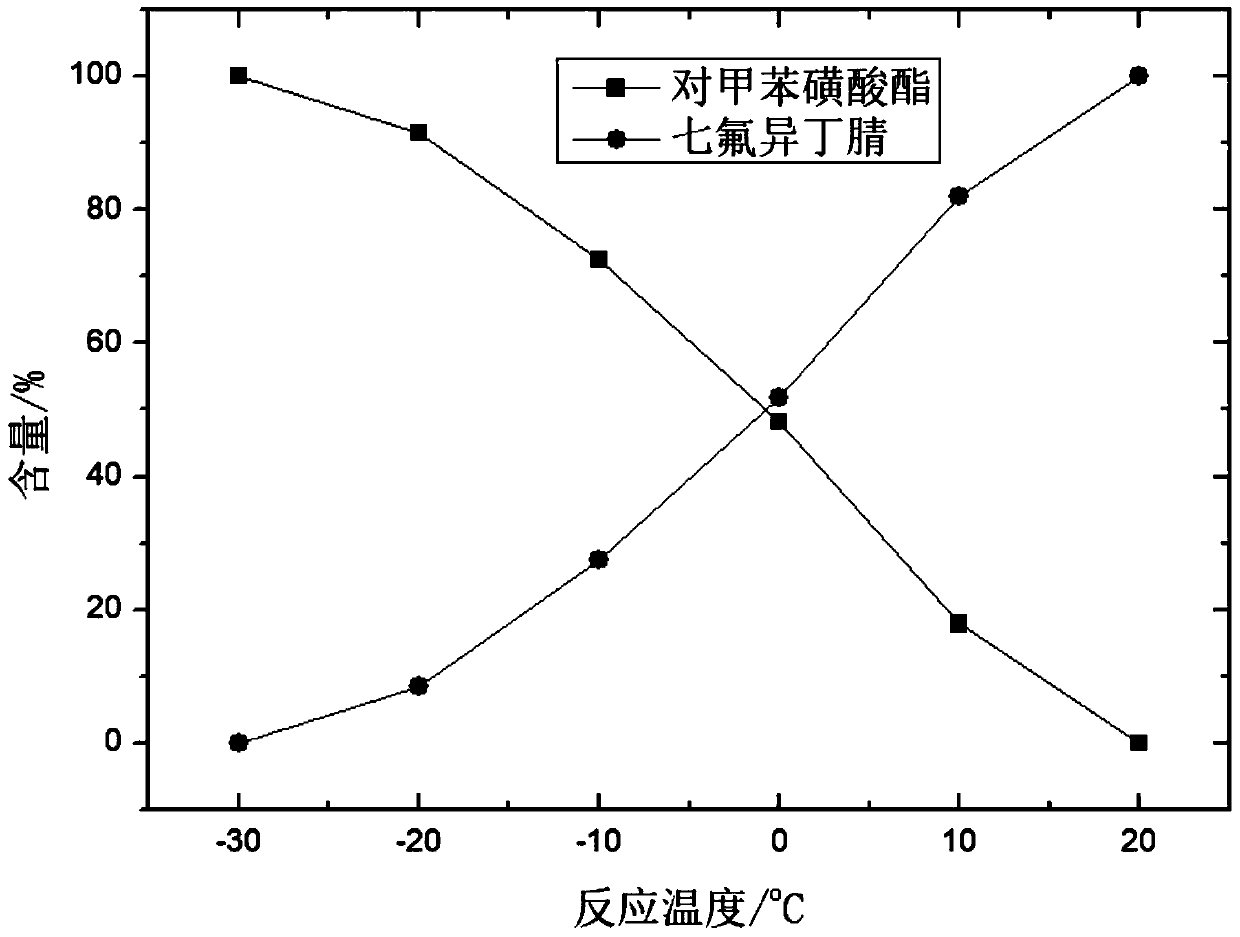
[0038] The autoclave used in the examples of the present application has a volume of 1 L, and the autoclave is equipped with stirring, a thermometer and a heat exchange jacket. Add 0.3 kg of methyl tert-butyl ether, 0.21 kg of p-toluenesulfonyl chloride, and 0.21 kg of heptafluoroisobutyramide into the autoclave, then cool down the autoclave, and add 0.12 kg of triethylamine. Incubate at -30°C for 1 hour; finally analyze the product of the autoclave, the product contains p-toluenesulfonate and the target product heptafluoroisobutyronitrile, and record the p-toluenesulfonate and target product heptafluoroisobutyronitrile Changes in content (deducting the content of other substances). The result is as figure 1 shown.
Embodiment 2
[0040] The embodiment of the present application provides the second preparation method of heptafluoroisobutyronitrile, the specific steps are as follows:
[0041] The autoclave used in the examples of the present application has a volume of 1 L, and the autoclave is equipped with stirring, a thermometer and a heat exchange jacket. Add 0.3 kg of methyl tert-butyl ether, 0.21 kg of p-toluenesulfonyl chloride, and 0.21 kg of heptafluoroisobutyramide into the autoclave, then cool down the autoclave, and add 0.12 kg of triethylamine. Incubate at -20°C for 1 hour; finally analyze the product of the autoclave, the product contains p-toluenesulfonate and the target product heptafluoroisobutyronitrile, and record the p-toluenesulfonate and target product heptafluoroisobutyronitrile Changes in content (deducting the content of other substances). The result is as figure 1 shown.
Embodiment 3
[0043] The embodiment of the present application provides the third preparation method of heptafluoroisobutyronitrile, the specific steps are as follows:
[0044] The autoclave used in the examples of the present application has a volume of 1 L, and the autoclave is equipped with stirring, a thermometer and a heat exchange jacket. Add 0.3 kg of methyl tert-butyl ether, 0.21 kg of p-toluenesulfonyl chloride, and 0.21 kg of heptafluoroisobutyramide into the autoclave, then cool down the autoclave, and add 0.12 kg of triethylamine. Incubate at -10°C for 1 hour; finally analyze the product of the autoclave, the product contains p-toluenesulfonate and the target product heptafluoroisobutyronitrile, and record the p-toluenesulfonate and target product heptafluoroisobutyronitrile Changes in content (deducting the content of other substances). The result is as figure 1 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com