Application of chrysin in in-vitro expansion of human hematopoietic stem cells
An in vitro amplification and artificial blood technology, applied in the field of cell culture, can solve problems such as poor quality, difficult hematopoietic reconstruction, and low success rate of engraftment, and achieve the effect of self-renewal promotion and self-renewal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A kind of small molecular compound chrysin (chrysin C2968), its structure is:
[0029]
Embodiment 2
[0031] A method for in vitro expansion of human hematopoietic stem cells, comprising the steps of:
[0032] Freshly isolated human cord blood CD34 + Cells were resuspended in culture medium (Gibco Iscove's Modified Dulbecco's Medium+10% FBS+100ng / mL SCF+100ng / mL TPO+100ng / mL Flt3L+1%P / S) to a cell concentration of 5×10 4 Cells / mL, then spread in a 96-well plate, each well is 190 μL of cell suspension, and then add 10 μL of chrysin (C2968) small molecule compound diluted with the same culture medium, chrysin (C2968) small molecule compound The final concentration was 2.5 μM. at 37°C, 5% CO 2 Cultured in a constant temperature incubator for 7 days.
Embodiment 3
[0033] Example 3: Cell Phenotype Analysis
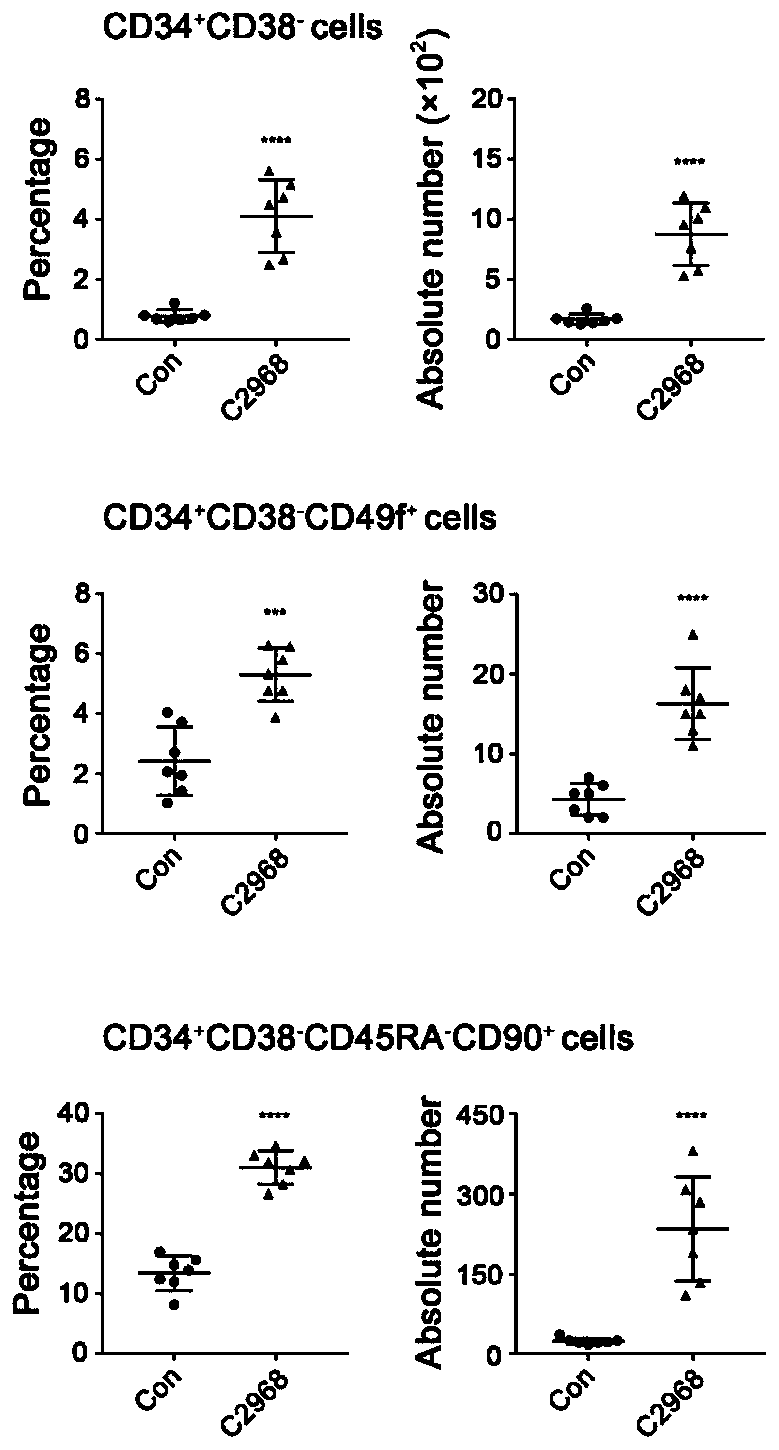
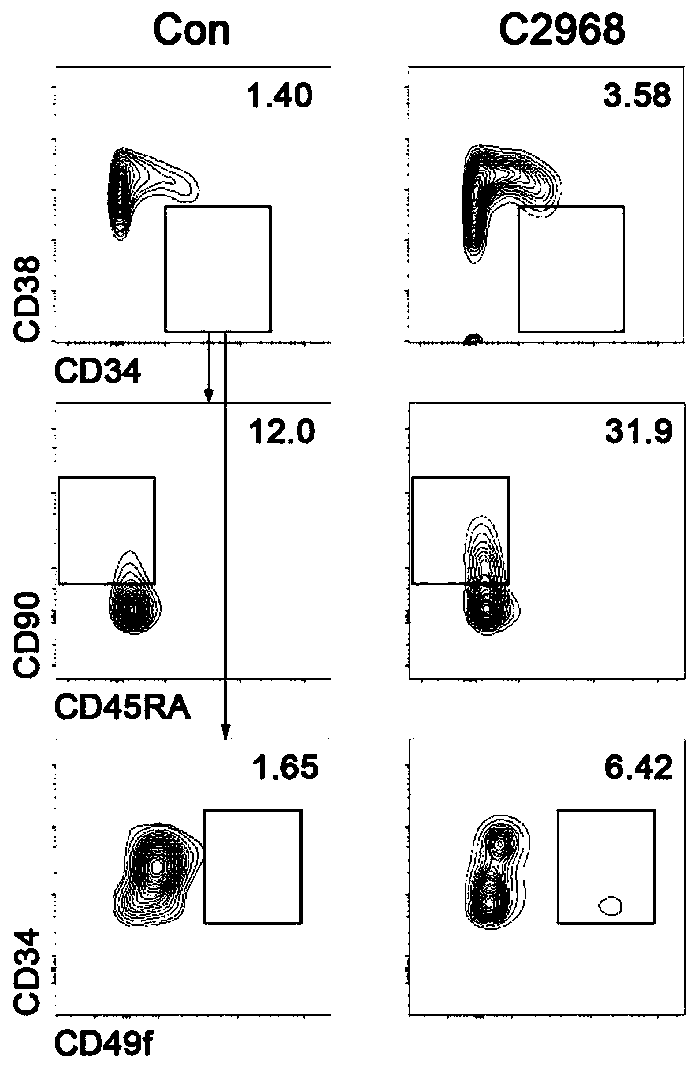
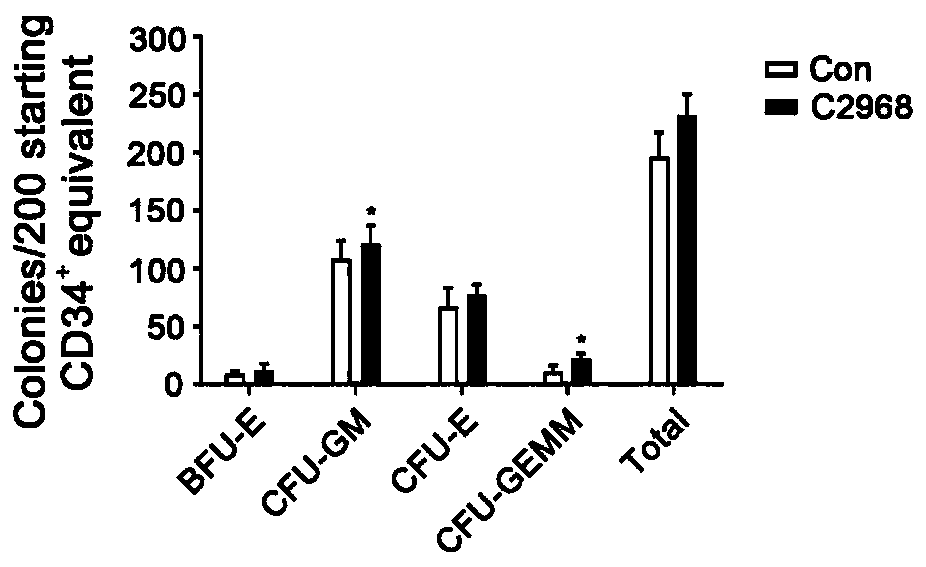
[0034] Using flow cytometry to detect the proportion and absolute number of CD34+CD38-, CD34+CD38-CD49f+, CD34+CD38-CD45RA-CD90+ cells to verify the effect of chrysin (C2968) small molecule compound on the expansion of hematopoietic stem cells in vitro, the results showed that poplar The C2968 small molecular compound has a significant effect on the expansion of hematopoietic stem cells in vitro.
[0035] Experimental method for detecting the effect of small molecular compounds of chrysin (C2968) on the expansion of human hematopoietic stem cells by flow cytometry:
[0036] ①Put the collected neonatal umbilical cord blood into a clean and sterile 200mL plasma bottle, add HES according to the volume ratio of hydroxyethyl starch (HES): umbilical cord blood = 4:1, blow and mix well, and let stand at room temperature for 40 Minutes to 1 hour to fully settle the red blood cells in the blood;
[0037] ②Use a 25mL disposable sterile pipet...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com