Application of ursane pentacyclic triterpenoids in preparing tumor radiotherapy sensitivity drugs
A tumor radiotherapy and compound technology, which is applied in the direction of anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve the problem of tumor radiotherapy resistance that is difficult to overcome, and achieve the effect of improving sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Example 1 Immunoprecipitation method to determine the inhibitory activity of compounds to specific deSUMOylated protease 1
[0030] Hela cells in logarithmic growth phase were inoculated on 25cm 2 Cell culture flasks were cultured overnight; after the cells completely adhered to the wall and recovered their shape, they were replaced with the medium containing ursolic acid or the test compound at a final concentration of 5 μM, and continued to culture for 24 hours; the medium was removed, and washed with PBS for two Digest with 0.25% trypsin, collect cells by centrifugation at 1500rpm for 5min; add 200μl RIPA lysate (with protease inhibitors PMSF and aprotinin added) to each sample, lyse on ice for 1h, shake and suspend once every 15min; 4°C Centrifuge at low temperature, 14000×g for 15 minutes, carefully absorb the supernatant (avoid absorbing the precipitate). Protein quantification was performed on the recovered supernatant.
[0031] Protein quantification was perfo...
Embodiment 2
[0045] Example 2 Cytotoxicity Experiment
[0046] Hela cells in the logarithmic growth phase were divided into 3×10 4 Cells / mL were inoculated in 96-well plates, 100 μL per well, and cultured overnight. After the cells were adhered to the wall and their morphology recovered under the microscope, the original medium was sucked off, washed with D-hanks buffer, and the drug solution of the compound to be tested was added to the medium without fetal bovine serum. The final concentrations were 0.7, 2.2, 6.6, 20μM, set three replicate wells for each group, and set the corresponding blank group, add 0.1% DMSO to the control group, set three replicate holes for each group, and set the corresponding blank group; at 37°C, 5% CO 2 Continue culturing for 72 hours under the same conditions, add 10 μL of CCK-8 detection solution to each well, and continue to incubate for 1 hour in a 37° C. incubator. Use a microplate reader to read the absorbance value (A) of each well at a wavelength of ...
Embodiment 53
[0054] Example 53 clone formation experiment
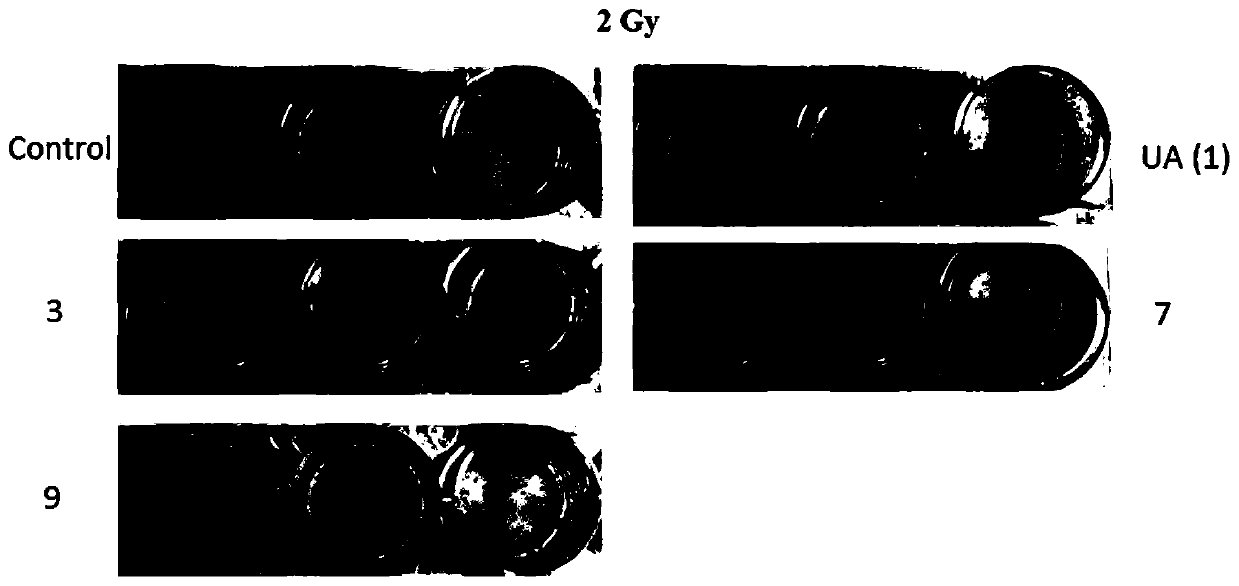
[0055] Hela cells in the logarithmic growth phase were digested with 0.25% trypsin and pipetted to form a single cell, and the cell suspension was diluted to an appropriate concentration. In the experiment, 1, 18, and 22 were used as experimental groups, and 5 groups of different irradiation doses (0Gy, 2Gy, 4Gy, 6Gy, 8Gy) were set for each group. Different numbers of cells were inoculated in different irradiation dose groups, and each dose of medicine in each group was set In three parallel experimental wells, 200 cells were inoculated per well in the 0Gy group and 2Gy group, 500 cells were inoculated in each well in the 4Gy group, 2000 cells were inoculated in each well in the 6Gy group, and 5000 cells were inoculated in each well in the 8Gy group. After inoculating the cells into a 24-well plate containing 500 μL of medium, pre-warm at 37°C, vibrate in the shape of a zigzag to disperse the cells evenly, and incubate overnight t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com