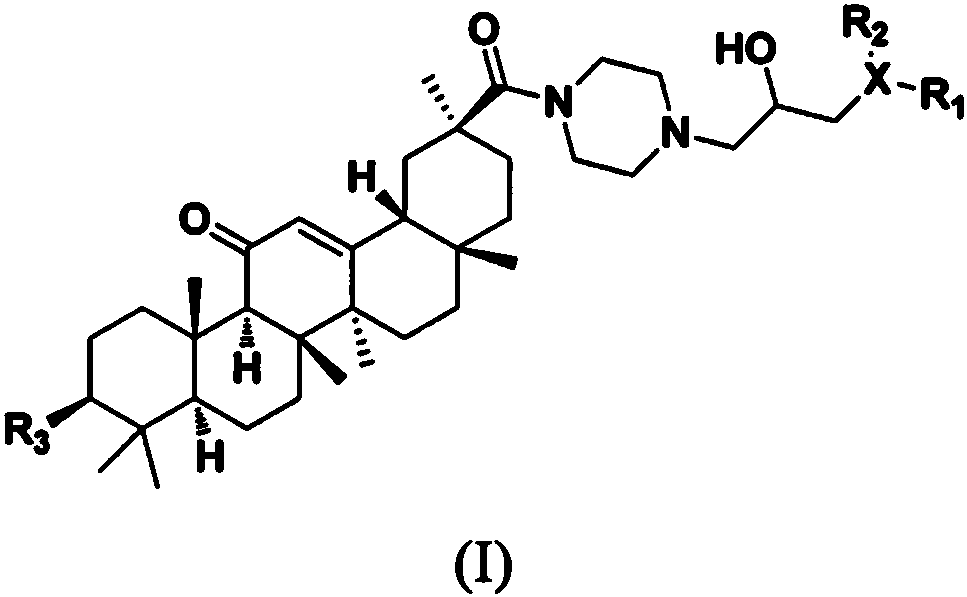
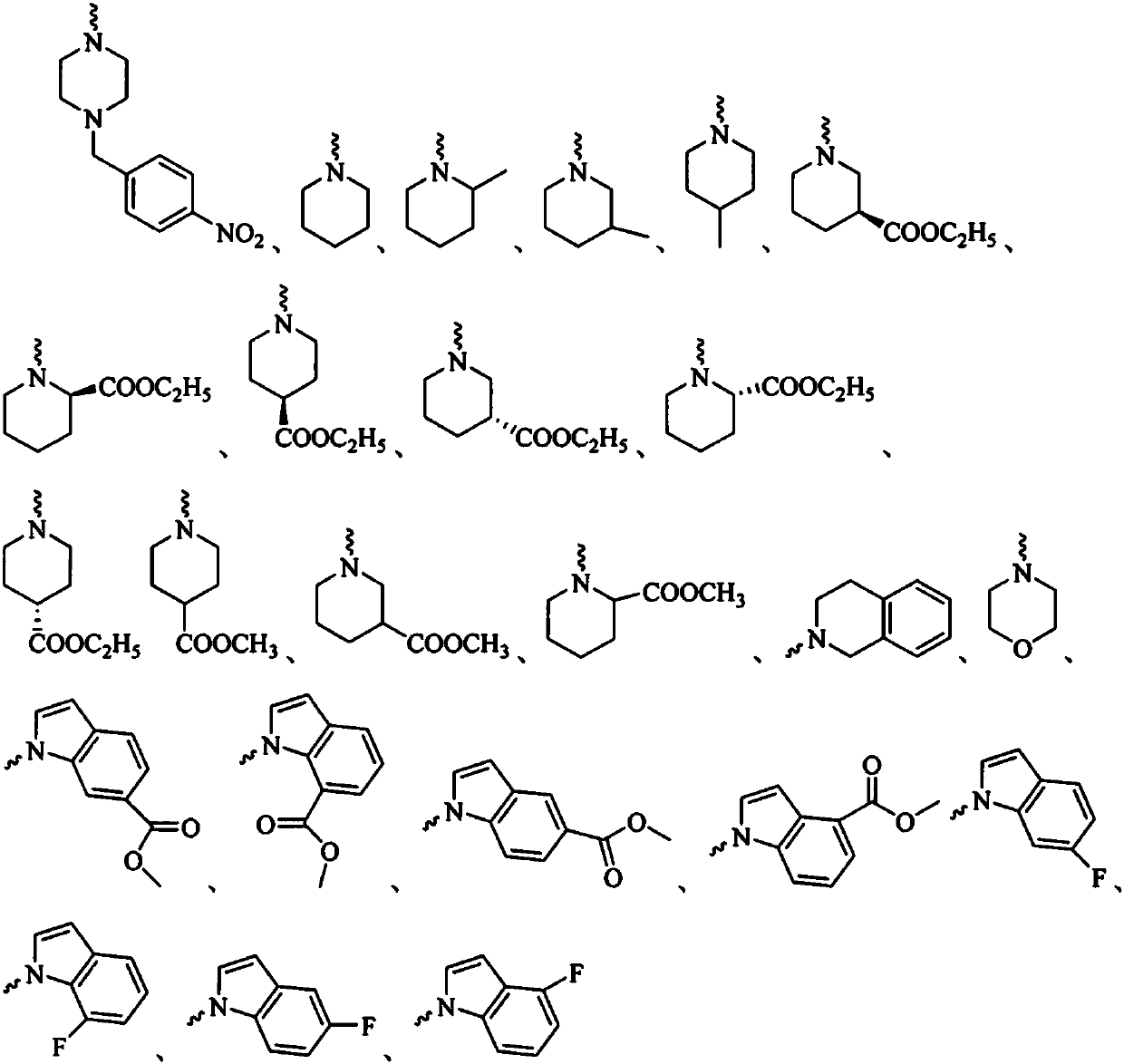
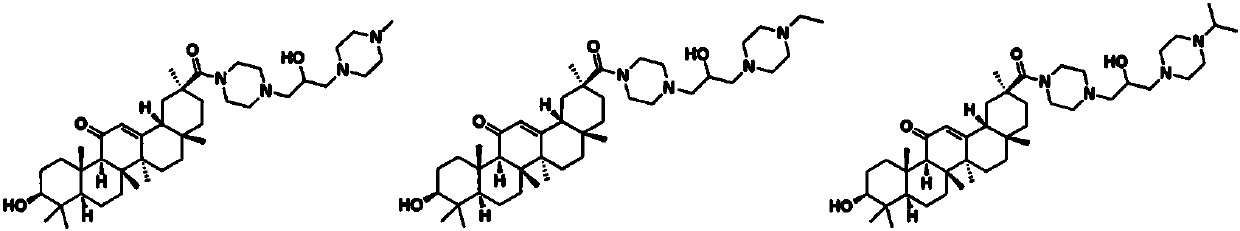
Glycyrrhetinic acid-piperazine compounds containing isopropanolamine substructure as well as preparation method and application thereof
A technology of propanolamine and glycyrrhetinic acid, applied in botany equipment and methods, steroids, applications, etc., can solve problems such as complex structure, difficult synthesis, easy volatility, and instability to light
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1: Preparation of intermediate 4-(oxyethylene-2-ylmethyl)-1-(18β-glycyrrhetinic acid group)-piperazine
[0060] Add 18β-glycyrrhetinic acid-30-piperazine (2.6mmol) into 20mL DMF solution dissolved with triethylamine (3.1mmol) and epibromohydrin (2.9mmol), react at room temperature for 12h, stop the reaction, extract with ethyl acetate , washed with saturated ammonium chloride, dried, precipitated, and column chromatographed to obtain a white solid with a yield of 66.4%. Its NMR data are: 1 H NMR (400MHz, CDCl 3 ) δ5.69 (s, 1H, 12-CH=C), 3.71-3.56 (m, 4H, 1'-NCH 2 ), 3.21 (dd, J=10.8, 5.3Hz, 1H, 3- CH OH), 3.13-3.04(m, 1H, 4'-OCH), 2.84-2.72(m, 3H, 1a-H+3'-N CH 2 CH), 2.66-2.40 (m, 5H, 2'-NCH 2 +5′a-CH 2 O), 2.35-2.17(m, 2H, 18-H+5'b-CH 2 O), 2.32(s, 1H, 9-H), 2.12-2.00(m, 2H, 16a-H+19a-H), 1.96(dd, J=13.6, 3.3Hz, 1H, 21a-H), 1.82 (td, J=13.5, 4.2Hz, 1H, 15a-H), 1.68-1.55(m, 5H, 6a-H+2-H+7a-H+21b-H), 1.51-1.34(m, 5H, 6b-H+7b-H+19b-H+22-H), 1.35(s, 3H, ...
Embodiment 2
[0061] Example 2: 1-(1-methylpiperazinyl)-3-(18β-glycyrrhetinic acid-30-piperazinyl)-2-hydroxypropanol
[0062] Add 4-(oxyethylene-2-ylmethyl)-1-(18β-glycyrrhetinyl)-piperazine (0.3mmol) into a solution of potassium carbonate (0.3mmol) and N-methylpiperazine (0.3 mmol) in 5 mL of isopropanol solution, reacted at 60°C for 24 h, then stopped the reaction, extracted with 30 mL of dichloromethane, washed with water, dried, precipitated, and column chromatographed to obtain a white solid with a yield of 74.9%.
[0063] The structure, H NMR spectrum and C NMR spectrum data of the synthesized 18β-glycyrrhetinic acid-30-piperazine compound containing isopropanolamine substructure are shown in Table 1, and the physicochemical properties are shown in Table 2.
[0064] Table 1 H NMR and C NMR data of the compounds
[0065]
[0066]
[0067]
[0068]
[0069]
[0070]
[0071]
[0072]
[0073]
[0074]
[0075]
[0076]
[0077]
[0078]
[0079...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com