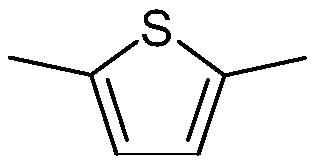
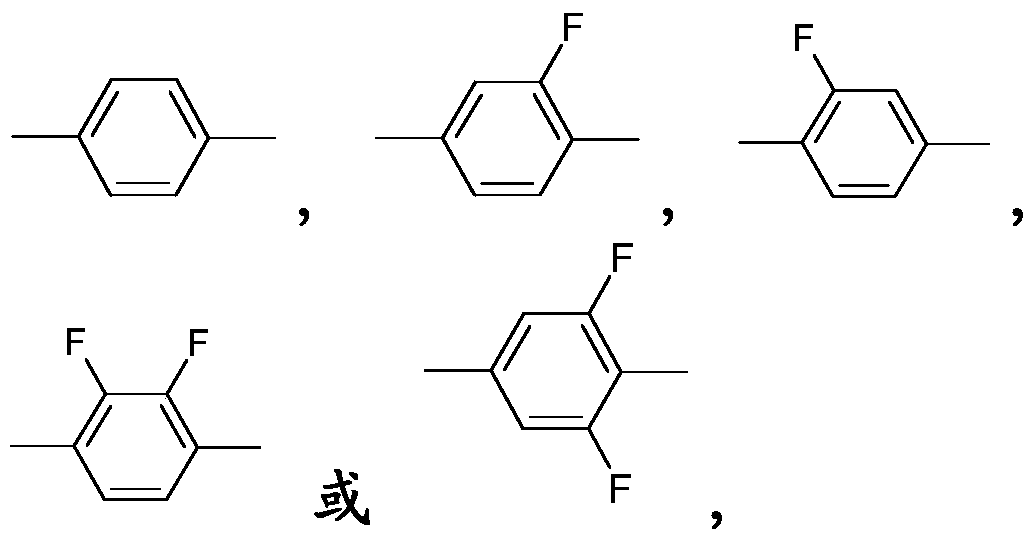
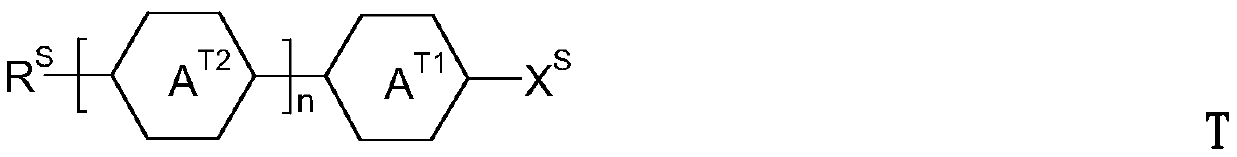Thiophene compound, liquid-crystalline medium and liquid-crystal display comprising the same
A liquid crystal medium and compound technology, applied in the field of liquid crystal displays, can solve the problems of low contrast, insufficient life, high operating voltage, etc., and achieve the effects of high positive dielectric anisotropy, good low temperature stability, and favorable capacitance threshold.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[1052] The following examples illustrate the invention without restricting it in any way. However, the physical properties make it clear to those skilled in the art what properties can be achieved and to what extent they can be adjusted. In particular, combinations of properties which can preferably be achieved are therefore well defined for the person skilled in the art.
[1053] The following abbreviations are used in the synthesis examples of this application:
[1054] BuLi n-butyllithium,
[1055] MTB ether tert-butyl methyl ether,
[1056] THF Tetrahydrofuran, and
[1057] dist. Distilled.
Synthetic example 1
[1058] Synthesis Example 1 (PUS-3-T)
[1059] Synthesis of 2-[2,6-difluoro-4-(4-propylphenyl)phenyl]-5-(trifluoromethyl)thiophene
[1060]
[1061] Step 1.1: 1,3-Difluoro-5-(4-propylphenyl)benzene
[1062]
[1063] (4-Propylphenyl)boronic acid (1) (7.0g, 42mmol), 1-bromo-3,5-difluoro-benzene (2) (8.1g, 40mmol), bis(dibenzylideneacetone) - A mixture of palladium(0) (50 mg, 0.87 μmol) and tris-(o-tolyl)phosphine (130 mg, 42 μmol) in acetone (120 mL) was heated to reflux under a nitrogen atmosphere, then sodium hydroxide solution (2N , 42mL, 84mmol). The reaction mixture was heated at reflux temperature for 2 hours. It was then cooled to ambient temperature and diluted with MTB ether and distilled water. The aqueous phase was separated and extracted with MTB ether. The combined organic phases were washed with distilled water and brine, dried over sodium sulfate and concentrated in vacuo. The residue was purified by silica gel chromatography (solvent heptane) to afford...
Synthetic example 2
[1072] Synthesis Example 2 (PUS-3-F)
[1073] Synthesis of 2-[2,6-difluoro-4-(4-propylphenyl)phenyl]-5-fluoro-thiophene
[1074]
[1075] Step 2.1: 2-[2,6-Difluoro-4-(4-propylphenyl)phenyl]-5-fluoro-thiophene
[1076]
[1077] 2-Bromo-1,3-difluoro-5-(4-propylphenyl)benzene (4) (5.3g, 17mmol), potassium carbonate (3.5g, 25mmol), tris(dibenzylidene-acetone )-Dipalladium(0) (80 mg, 87 μmol) and CataCXium A (55 mg, 153 μmol) in THF (80 mL) and water (18 mL) were heated to reflux under a nitrogen atmosphere, then 2-(5- A solution of fluoro-2-thienyl)-4,4,5,5-tetramethyl-1,3-dioxaborolane (7) (4.1 g, 18 mmol) in THF (20 ml). The reaction mixture was heated at reflux temperature for 2 hours. It was then cooled to ambient temperature and diluted with MTB ether and distilled water. The aqueous phase was separated and extracted with MTB ether. The combined organic phases were washed with distilled water and brine, dried (sodium sulfate) and concentrated in vacuo. The residue...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Clear point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com