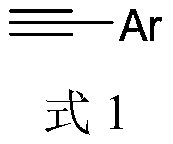Environmental-friendly preparation method of N-methyl-2-cyano-3-arylpyrrole compound
An arylpyrrole and compound technology, which is applied in the field of green preparation of N-methyl-2-cyano-3-arylpyrrole compounds, can solve the problems of low reaction efficiency, low atom utilization rate and the like, and achieves high reaction efficiency , The reaction conditions are environmentally friendly and green, and the effect of high reaction area selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~14
[0055] Following examples 1~14 all react by following reaction equation:
[0056]
[0057] The specific operation steps are: in a 50mL reaction tube, sequentially add terminal aryne (40mmol), trimethylnitrile silane (40mmol, 3.97g), N,N-dimethylformamide (40mmol, 2.93g), iodine ( 0.8 mmol, 1 g), the resulting mixture was stirred and reacted at 80°C for 2 hours. After the reaction, add 30ml of saturated sodium sulfite for washing, extract 3 times with 30mL of ethyl acetate, combine the extracts, concentrate the extracts in vacuo, dry in vacuo and calculate the weight.
Embodiment 1
[0059] raw material: Target product:
[0060] 1-methyl-3-phenyl-1H-pyrrole-2-carbonitrile:
[0061] Yield: 94%.
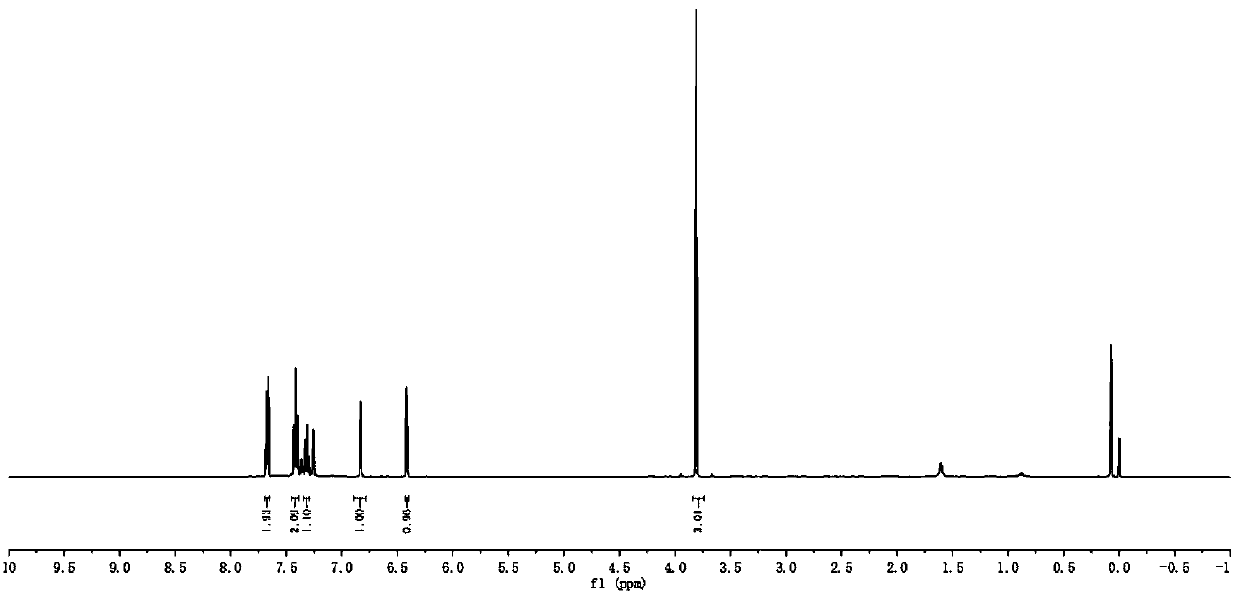
[0062] 1 H NMR (500MHz, CDCl 3 )δ7.69–7.65 (m, 2H), 7.41 (t, J = 7.7Hz, 2H), 7.31 (t, J = 7.4Hz, 1H), 6.83 (d, J = 2.6Hz, 1H), 6.41 ( d,J=2.7Hz,1H),3.81(s,3H).
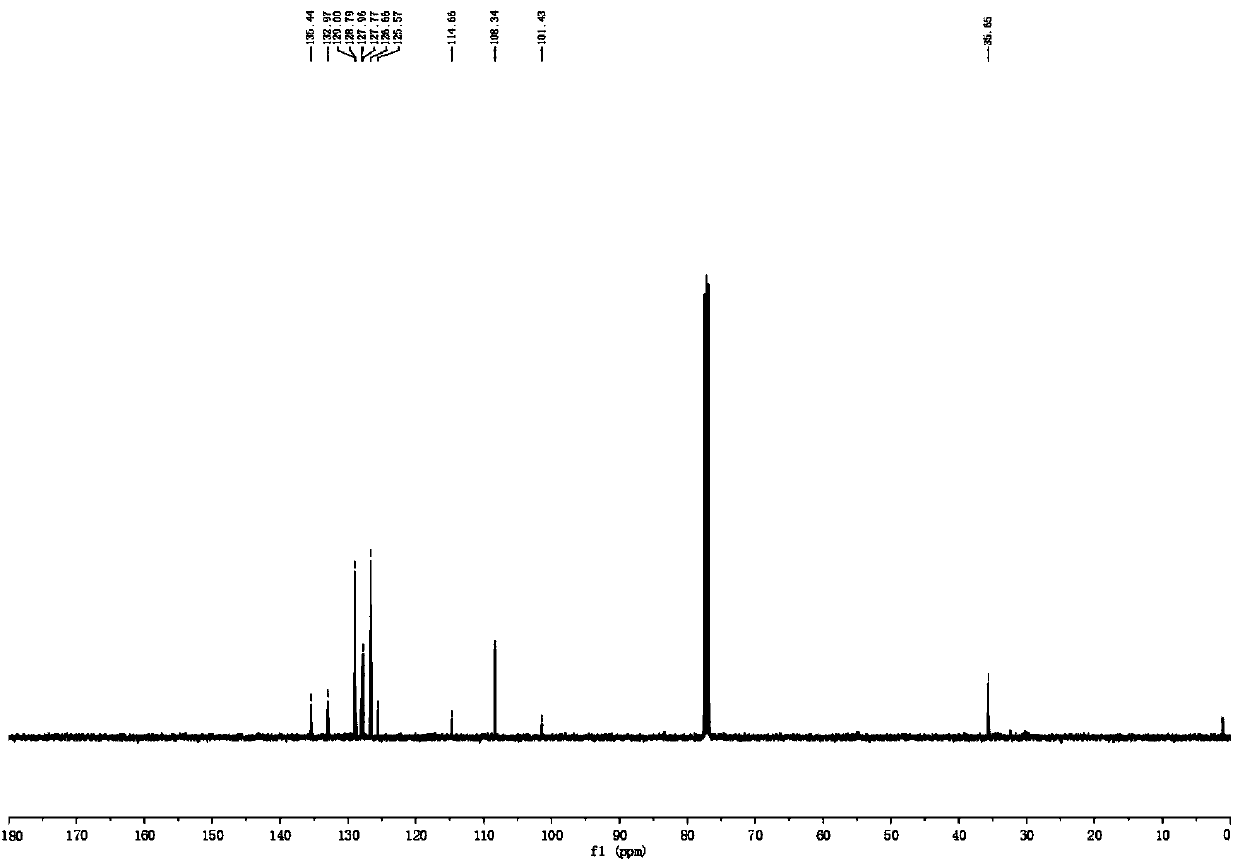
[0063] 13 C NMR (126MHz, CDCl 3 )δ135.44, 132.97, 128.79, 127.96, 127.77, 126.66, 114.66, 108.34, 101.43, 35.65.
Embodiment 2
[0065] raw material: Target product:
[0066] 1-methyl-3-(p-tolyl)-1H-pyrrole-2-carbonitrile:
[0067] Yield: 95%.
[0068] 1 H NMR (500MHz, CDCl 3 )δ7.57(d, J=8.0Hz, 2H), 7.22(d, J=7.8Hz, 2H), 6.82(d, J=2.4Hz, 1H), 6.39(d, J=2.5Hz, 1H) ,3.80(s,3H),2.37(s,3H).
[0069] 13 C NMR (126MHz, CDCl 3 )δ137.65, 135.56, 130.13, 129.69, 127.89, 126.56, 114.78, 108.18, 101.25, 35.61, 21.35.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com