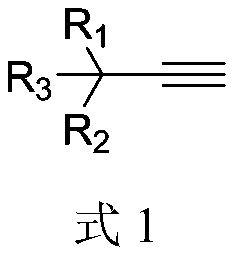
1-methyl-2-cyano-3-aliphatic substituted pyrrole compound synthesis method
A synthetic method and aliphatic technology, applied in the direction of organic chemistry, can solve the problems of low Ecoscale index, impossibility of industrial promotion and application, and low atom utilization rate, so as to improve environmental index, improve atom utilization rate and efficiency, and increase yield and the effect of purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~3
[0055] Following examples 1~3 all react by following reaction equation:
[0056]
[0057] The specific operation steps are: in a 50mL pressure-resistant tube, add octyne (30mmol), trimethylcyanosilane (30mmol, 2.97g), N,N-dimethylformamide (30mmol, 2.19g), iodine ammonium chloride (9mmol, 1.30g), and the resulting mixture was stirred and reacted at 100°C for about 3 hours. After the reaction, add 15ml of saturated sodium sulfite for washing, extract 3 times with 15mL of ethyl acetate, combine the extracts, concentrate the extracts in vacuo, dry in vacuo and calculate the weight.
Embodiment 1
[0059] raw material: Target product:
[0060] 3-hexyl-1-methyl-1H-pyrrole-2-carbonitrile:
[0061] Yield: 92%.
[0062] Yellowish green oil;
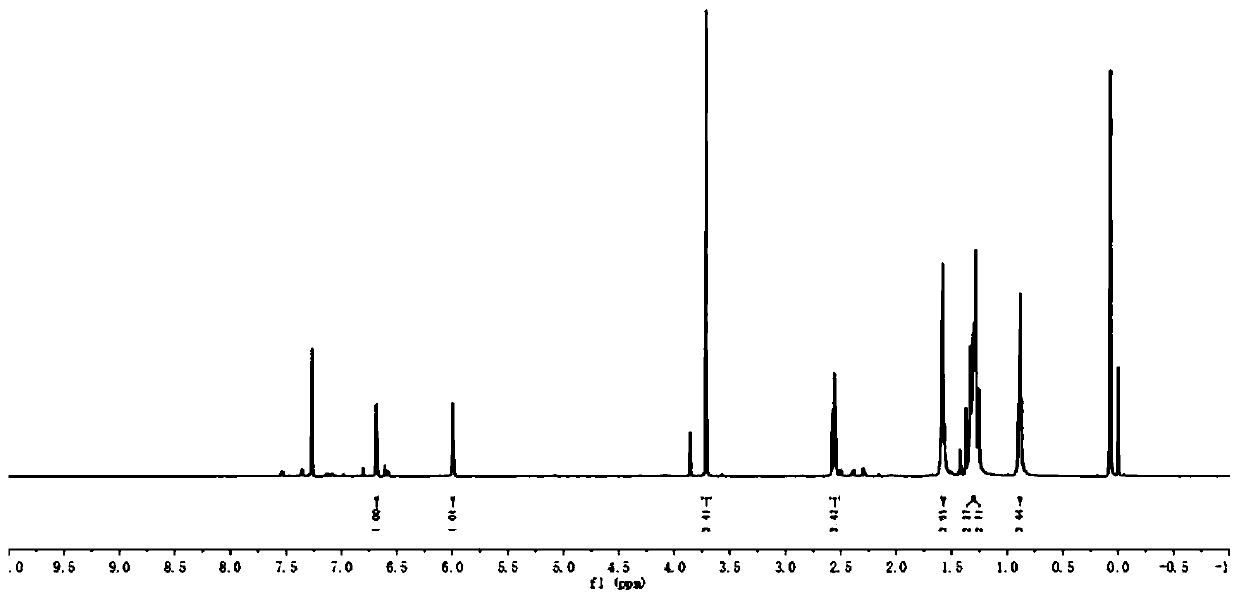
[0063] 1 H NMR (500MHz, CDCl 3 )δ6.68(d, J=2.5Hz, 1H), 5.99(d, J=2.5Hz, 1H), 3.71(s, 3H), 2.56(t, J=7.7Hz, 2H), 1.59-1.50( m,4H),1.31-1.30(m,2H),1.30-1.29(m,2H),0.89(t,J=6.7Hz,3H).
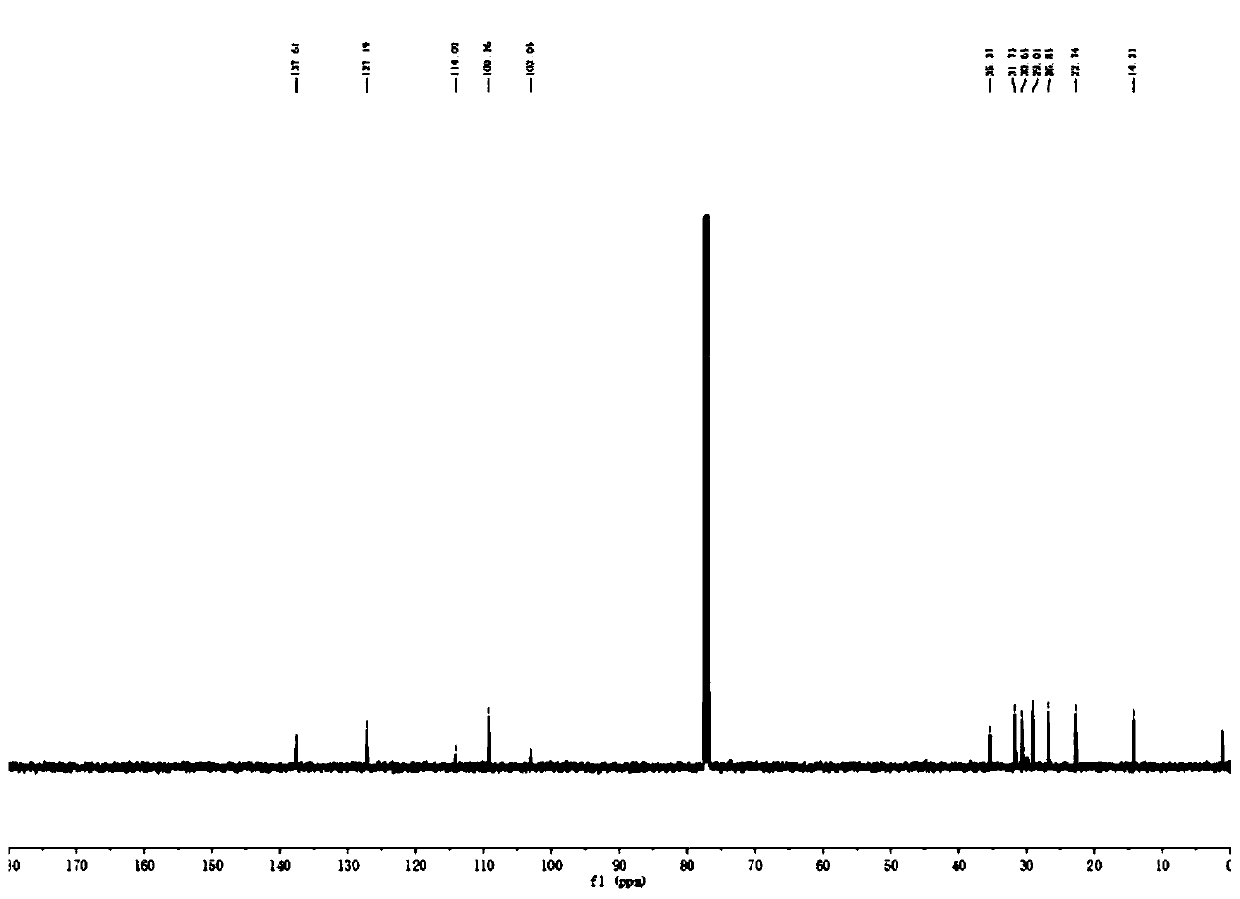
[0064] 13 C NMR (126MHz, CDCl 3 )δ137.61, 127.19, 114.09, 109.26, 103.05, 35.37, 31.73, 30.65, 29.01, 26.85, 22.74, 14.23.
[0065] IR (neat, cm –1 )ν2970, 2260, 1555, 1320, 1041, 886, 761, 738.
[0066] HRMS (ESI) calcd for C 9 h 19 N 2 [M+H] + :191.1543, found 191.1546.
Embodiment 2
[0068] raw material: Target product:
[0069] 3-benzyl-1-methyl-1H-pyrrole-2-carbonitrile:
[0070] Yield: 82%.
[0071] 1 H NMR (500MHz, CDCl 3 )δ7.29(t, J=7.5Hz, 2H), 7.25-7.19(m, 3H), 6.69 (d, J=2.4Hz, 1H), 5.96(d, J=2.4Hz, 1H), 3.91( s,2H),3.73(s,3H).
[0072] 13 C NMR (126MHz, CDCl 3 )δ140.23, 135.72, 128.67, 128.64, 127.49, 126.41, 113.81, 109.81, 103.30, 35.46, 33.16.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com