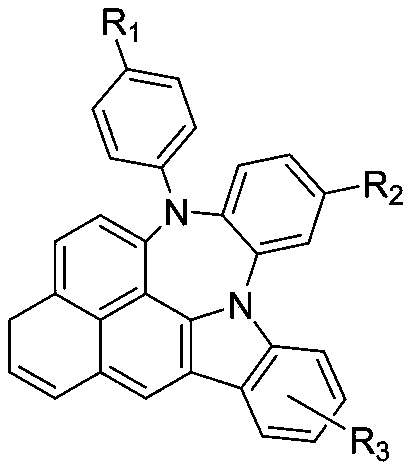
Nitrogen-containing fused-heterocyclic compound and preparation method thereof
A technology of compound and fused heterocycle, which is applied in the field of OLED materials, can solve the problems of different performances, and achieve the effect of large steric hindrance, difficult rotation, and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
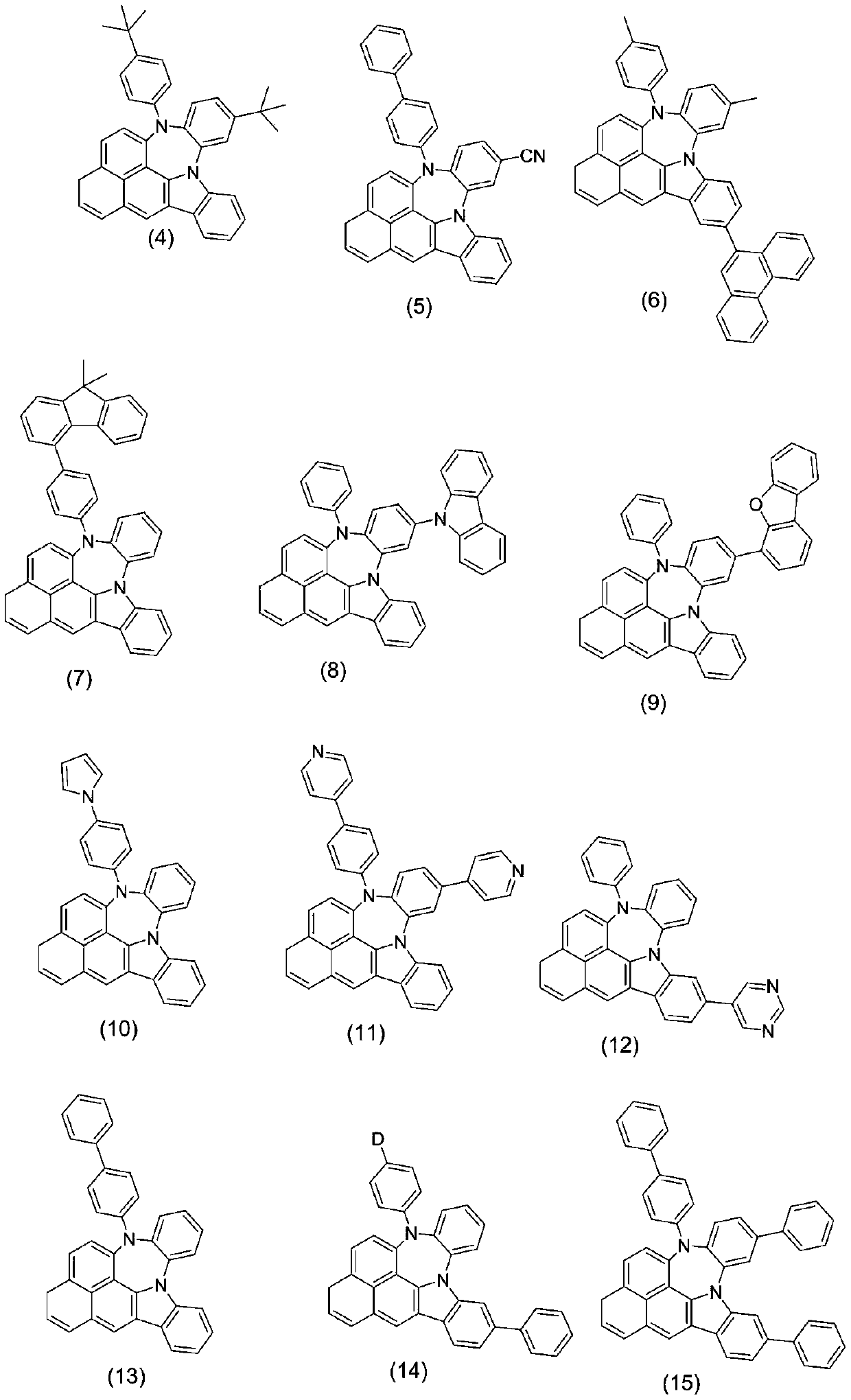
Image
Examples
Embodiment 1
[0027]
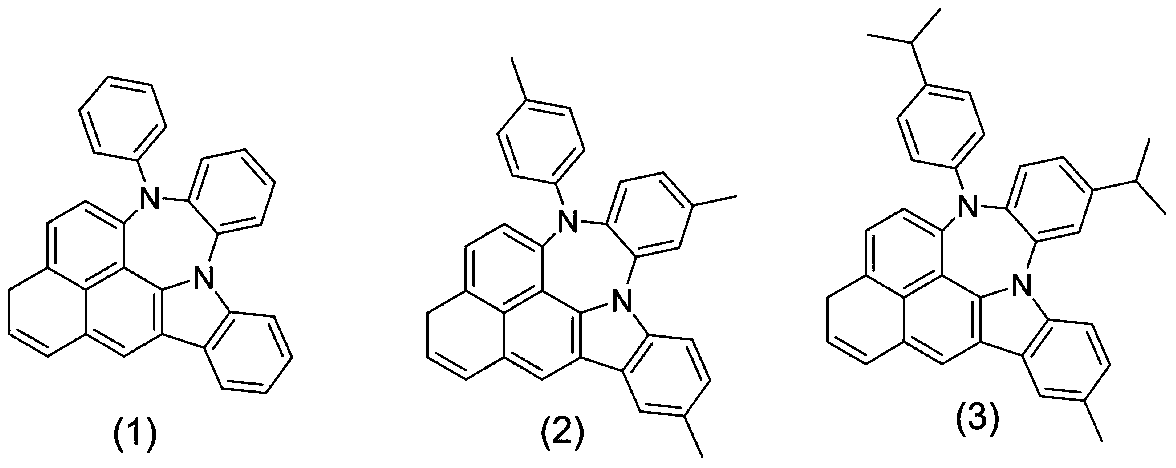
[0028] The synthetic method of nitrogen-containing condensed heterocyclic compound (1) is as follows:
[0029]
[0030] Under nitrogen protection with a flow rate of 20sccm, compound I (10.0g, 333.02g / mol, 30mmol), compound II (1.1eq, 8.16g, 247g / mol, 33.03mmol), sodium tert-butoxide (4eq, 11.54g, 96.1g / mol, 120.11mmol), tris(dibenzylideneacetone)dipalladium (0.05eq, 1.37g, 915g / mol, 1.5mmol), tri-tert-butylphosphine (0.05eq, 0.3g, 202.32g / mol , 1.5mol), toluene (100g) into the reaction flask, after the addition was completed, the temperature was raised to reflux and stirred for 5 hours, after the reaction was completed and cooled to room temperature, 100mL of water was added and stirred for 10 minutes, and the resulting mixture was filtered through diatomaceous earth to obtain an organic phase , the organic phase was dried with anhydrous magnesium sulfate and then spin-dried to obtain a crude product. The crude product was dissolved in dichloromethane and metha...
Embodiment 2
[0032]
[0033] The synthetic method of nitrogen-containing condensed heterocyclic compound (4) is as follows:
[0034]
[0035] Under nitrogen protection with a flow rate of 10sccm, compound Ⅰ (10.0g, 333.02g / mol, 30mmol), compound Ⅱ (1.1eq, 11.86g, 359.12g / mol, 33.03mmol), sodium tert-butoxide (4eq, 11.54g , 96.1g / mol, 120.11mmol), tris(dibenzylideneacetone) dipalladium (0.05eq, 1.37g, 915g / mol, 1.5mmol), tri-tert-butylphosphine (0.05eq, 0.3g, 202.32g / mol, 1.5mol), toluene (100g) were added to the reaction flask, after the addition was completed, the temperature was raised to reflux and stirred for 5 hours, after the reaction was completed and cooled to room temperature, 100 mL of water was added and stirred for 10 minutes, and the obtained mixture was filtered through diatomaceous earth and separated to obtain organic phase, the organic phase was dried with anhydrous magnesium sulfate and spin-dried to obtain a crude product. The crude product was dissolved in dichlo...
Embodiment 3
[0037]
[0038] The synthetic method of nitrogen-containing condensed heterocyclic compound (5) is as follows:
[0039]
[0040]Under nitrogen protection with a flow rate of 50sccm, compound I (10.0g, 333.02g / mol, 30mmol), compound II (1.1eq, 11.49g, 348.03g / mol, 33.03mmol), sodium tert-butoxide (4eq, 11.54g , 96.1g / mol, 120.11mmol), tris(dibenzylideneacetone) dipalladium (0.05eq, 1.37g, 915g / mol, 1.5mmol), tri-tert-butylphosphine (0.05eq, 0.3g, 202.32g / mol, 1.5mol), toluene (150g) were added to the reaction flask, after the addition was completed, the temperature was raised to reflux and stirred for 5 hours, after the reaction was completed and cooled to room temperature, 100 mL of water was added and stirred for 10 minutes, and the resulting mixture was filtered through diatomaceous earth and separated to obtain organic phase, the organic phase was dried with anhydrous magnesium sulfate and then spin-dried to obtain a crude product. The crude product was dissolved in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com