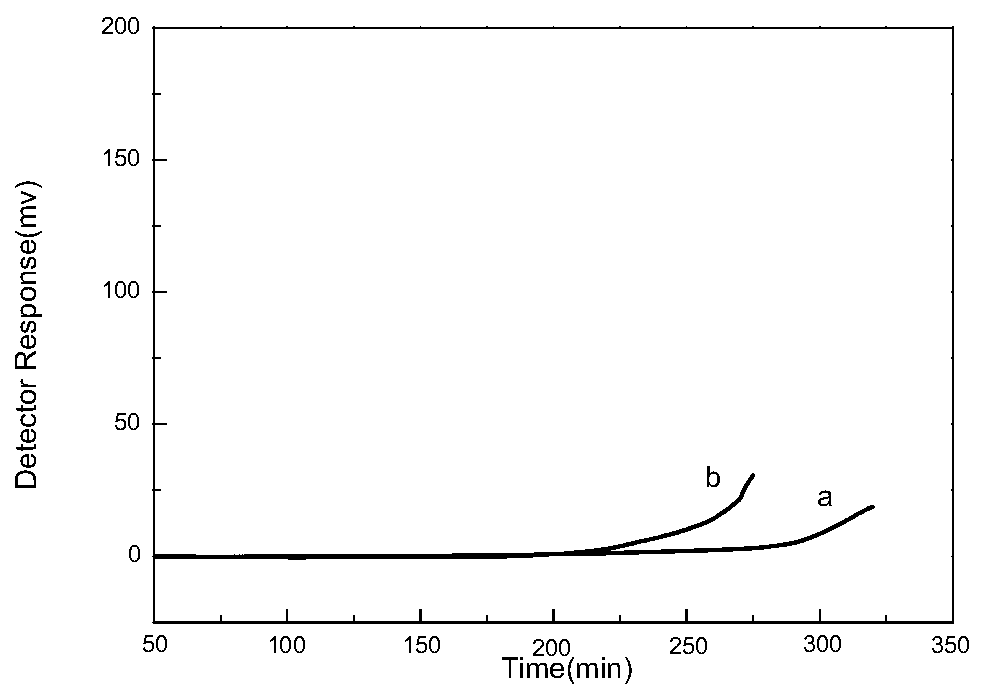
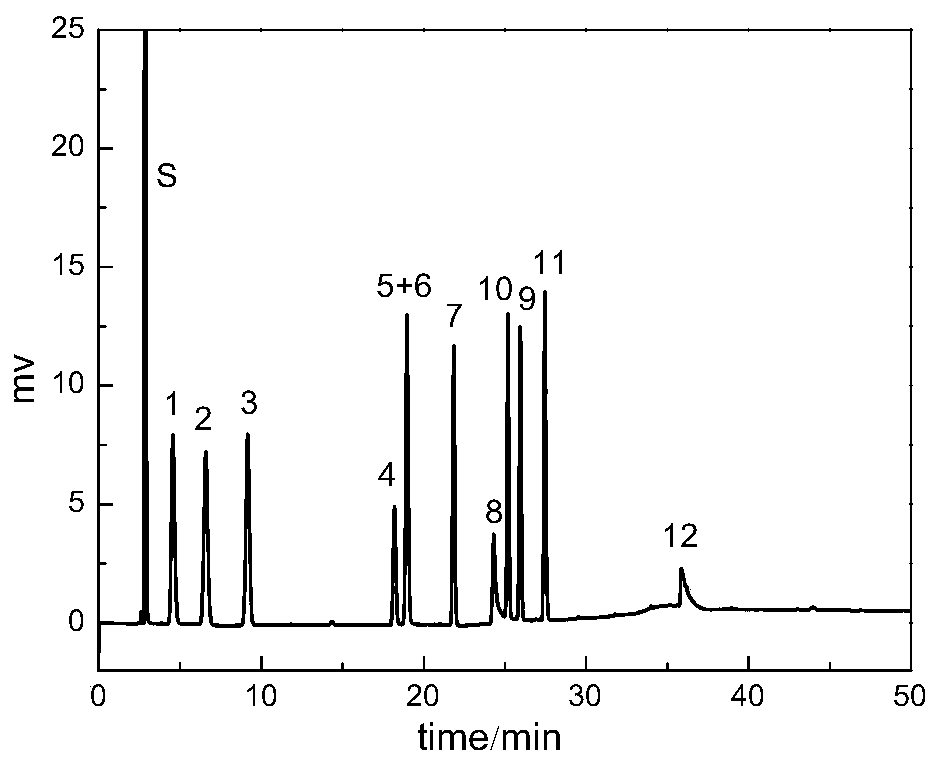
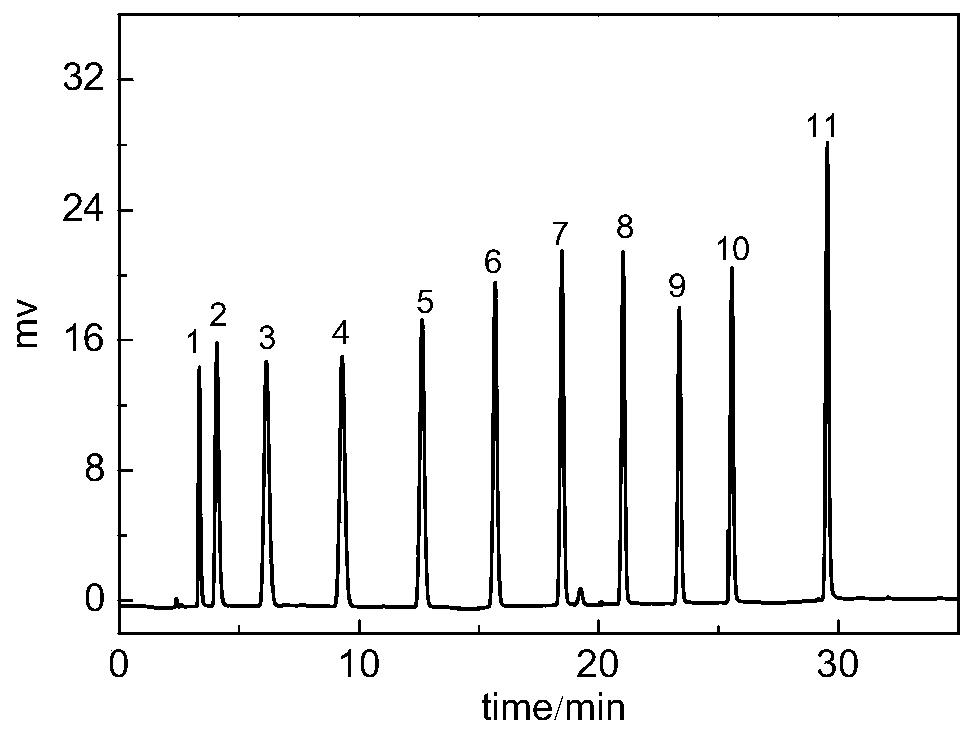
Dicationic liquid chromatographic stationary phase and preparation method and application thereof
A liquid stationary phase and double cation technology, applied in the field of ionic liquid chromatography stationary phase materials, to achieve good thermal stability, improved wetting ability, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] R in the synthesized dicationic liquid stationary phase in this embodiment is C 1 , whose structural formula is:
[0058]
[0059] The preparation method is as follows:
[0060] Synthesis of Dication Liquid Iodine Salt (MM(mim) 2 -(NTf 2 ) 2 ), adding 2:1 methylimidazole (8.21g) and 1,3-bis(iodomethyl)tetramethyldisiloxane (18.35g) in the reaction flask, adding 50mL of anhydrous acetonitrile solvent, reflux at 80°C, and react under nitrogen atmosphere for 72h. The solvent was distilled off under reduced pressure, and the unreacted substance was extracted with three equal parts of ethyl acetate, and dried to obtain a light yellow viscous compound, which is the dicationic liquid iodine salt. Synthesis of Dicationic Liquid Ditrifluoromethanesulfonylimide Salt (MM(mim) 2 -(NTf 2 ) 2 ), 0.01 mol of iodide salt was dissolved in 20 mL of deionized water, and 5.742 g of two molar equivalents of lithium bistrifluoromethanesulfonimide dissolved in 10 mL of deionized wa...
Embodiment 2
[0062] R in the synthesized dicationic liquid stationary phase in this embodiment is C 4 , whose structural formula is:
[0063]
[0064] The preparation method is as follows:
[0065] Synthesis of Dication Liquid Iodine Salt (MM(bim) 2 -I 2 ), adding butylimidazole (12.42g) and 1,3-bis(iodomethyl)tetramethyldisiloxane (18.35g) with a molar ratio of 2:1 in the reaction flask, adding 50mL of anhydrous acetonitrile for solvent, reflux at 80°C, and react under nitrogen atmosphere for 72h. The solvent was distilled off under reduced pressure, and the unreacted substance was extracted with three equal parts of ethyl acetate, and dried to obtain a light yellow viscous compound, which is the dicationic liquid iodine salt. Synthesis of Dicationic Liquid Bistrifluoromethanesulfonylimide Salt (MM(bim) 2 -(NTf 2 ) 2 ), 0.01 mol of iodide salt was dissolved in 20 mL of deionized water, and 5.742 g of two molar equivalents of lithium bistrifluoromethanesulfonimide dissolved in 10...
Embodiment 3
[0067] R in the dicationic liquid stationary phase synthesized in this embodiment is benzyl, and its structural formula is:
[0068]
[0069] The preparation method is as follows:
[0070] Synthesis of Dication Liquid Iodine Salt (MM(benzim) 2 -I 2 ), adding 2:1 benzyl imidazole (15.82g) and 1,3-bis(iodomethyl)tetramethyldisiloxane (18.35g) in the reaction flask, adding 50mL of anhydrous acetonitrile solvent, reflux at 80°C, and react under nitrogen atmosphere for 72h. The solvent was distilled off under reduced pressure, and the unreacted substance was extracted with three equal parts of ethyl acetate, and dried to obtain a light yellow viscous compound, which is the dicationic liquid iodine salt. Synthesis of Dicationic Liquid Ditrifluoromethanesulfonylimide Salt (MM(benzim) 2 -(NTf 2 ) 2 ), 0.01 mol of iodide salt was dissolved in 30 mL of methanol, and 5.742 g of two molar equivalents of lithium bistrifluoromethanesulfonylimide dissolved in 10 mL of deionized wate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com