Meridianin derivative as well as preparation and application thereof in prevention and treatment of plant virus and bacterial diseases
A technology for plant virus diseases and plant pathogens, which is applied in the fields of chemicals for biological control, botanical equipment and methods, applications, etc., which can solve the problem of increasing NFAT time, the biological activity of derivatives has not been systematically studied, and has not been systematically studied. research and reports on the issues, to achieve the effect of good anti-plant virus and fungus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
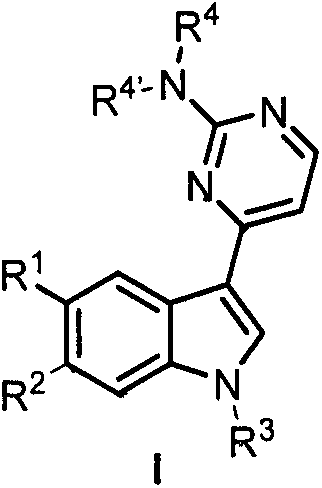
[0051] Embodiment 1: the synthesis of Meridianin derivatives I-1 and I-2
[0052] I-1: In a 100mL round bottom flask, dissolve meridianin C (0.2g, 0.7mmol) in acetonitrile, and add Boc 2 O (0.18g, 0.84mmol), DMAP (0.07mmol, 0.01g), react at room temperature for two hours. After the reaction is complete, remove the solvent, add water, extract with dichloromethane, wash the organic phase with water, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. Crude column chromatography (V 氯甲烷 :V 甲醇 =100:1→V 二氯甲烷 :V 甲醇 =20:1) to obtain 0.18 g of light yellow solid with a yield of 67% and a melting point of 171-173°C. 1 H NMR (400MHz, DMSO-d 6 )δ8.86(d, J=1.6Hz, 1H, Ar-H), 8.50(s, 1H, Ar-H), 8.25(d, J=5.2Hz, 1H, Ar-H), 8.07(d, J=9.2Hz, 1H, Ar-H), 7.56(dd, J=8.8, 1.6Hz, 1H, Ar-H), 7.21(d, J=5.2Hz, 1H, Ar-H), 6.77(s, 2H, NH 2 ), 1.67(s, 9H, C(CH 3 ) 3 ). 13 C NMR (100MHz, DMSO-d 6 )δ164.0, 160.7, 158.6, 148.9, 134.7, 129.8, 129.2, 128.1, 125.8, 118.1, 117...
Embodiment 2
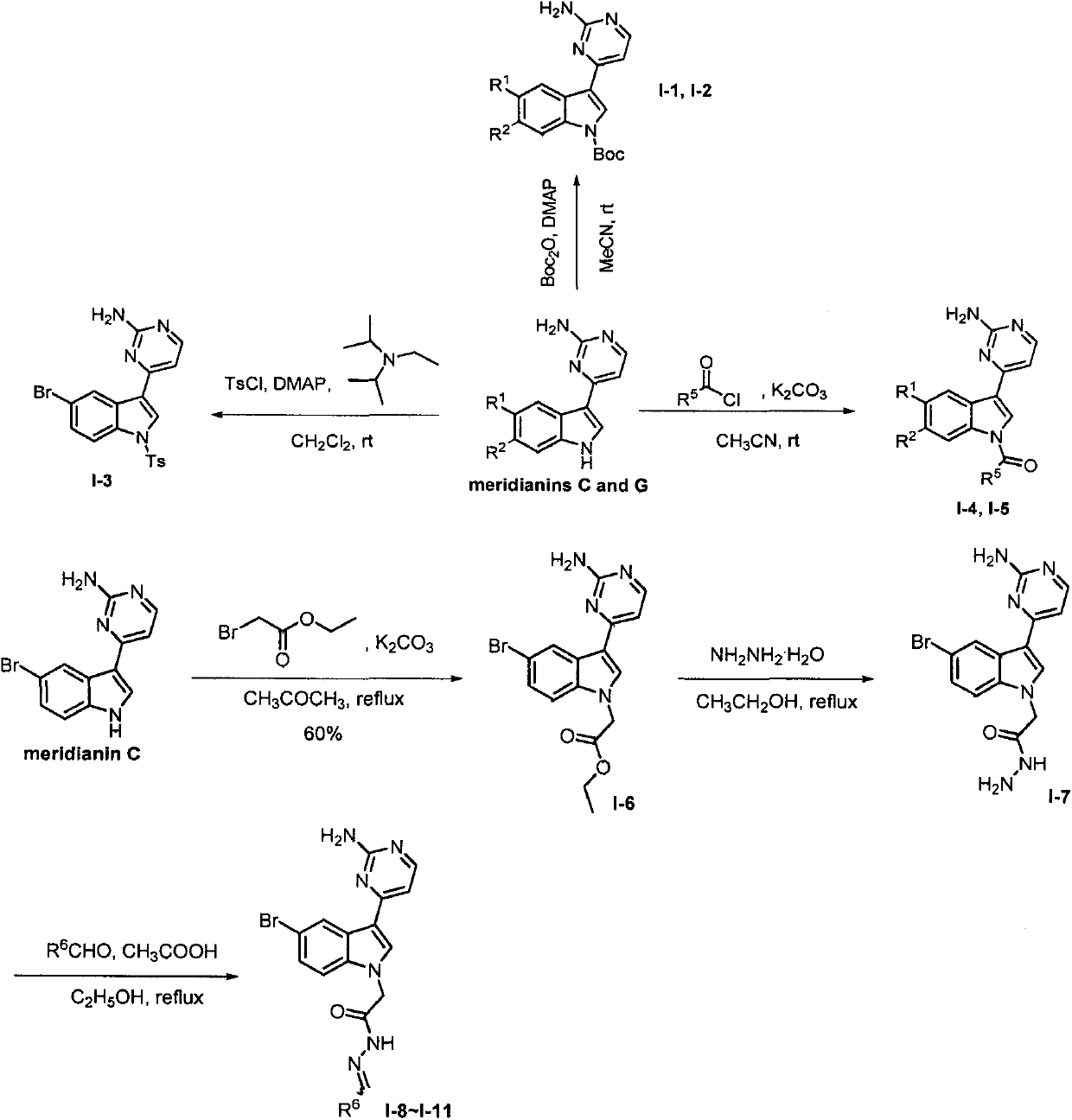
[0054] Embodiment 2: the synthesis of Meridianin derivatives I-3
[0055] In a 100mL round bottom flask, the natural product meridianin C (0.5g, 1.7mmol) was dissolved in dry dichloromethane, DMAP (0.01g, 0.085mmol), p-toluenesulfonyl chloride (0.33g, 1.87 mmol), N, N-diisopropylethylamine (0.33g,, 2.55mmol), stirred at room temperature for 20h. After the reaction is complete, add 10% dilute hydrochloric acid to the system, extract with dichloromethane, wash once with water and once with saturated brine, dry over anhydrous sodium sulfate, and concentrate under reduced pressure. Crude column chromatography (V 二氯甲烷 :V 甲醇 =100:1→V 二氯甲烷 :V 甲醇 =20:1) to obtain 0.5 g of a light yellow solid with a yield of 62% and a melting point of 216-217°C. 1 H NMR (400MHz, DMSO-d 6 )δ8.83(d, J=2.0Hz, 1H, Ar-H), 8.73(s, 1H, Ar-H), 8.26(d, J=5.2Hz, 1H, Ar-H), 7.98(d, J=8.4Hz, 2H, Ar-H), 7.94(d, J=8.8Hz, 1H, Ar-H), 7.57(dd, J=8.8, 2.0Hz, 1H, Ar-H), 7.43(d, J=8.4Hz, 2H, Ar-H), 7.26(d, J=5.2H...
Embodiment 3
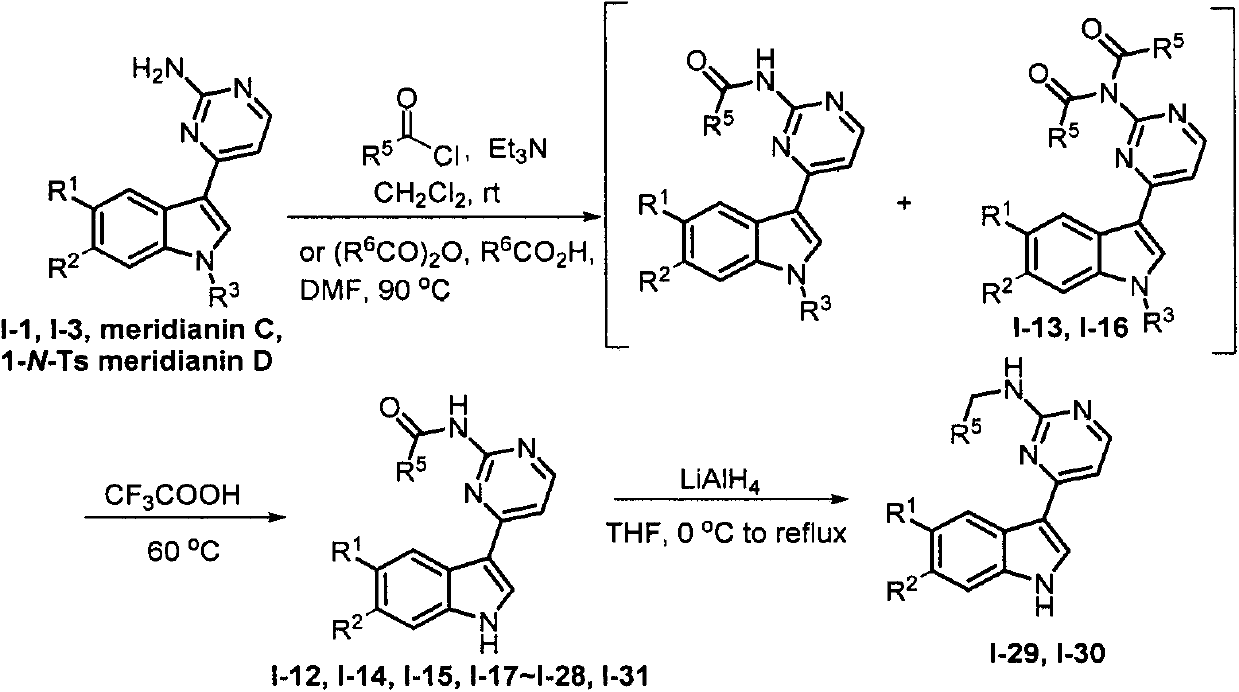
[0056] Embodiment 3: the synthesis of Meridianin derivatives I-4 and I-5
[0057] I-4: In a 100mL round bottom flask, dissolve meridianin D (0.5g, 1.7mmol) in acetonitrile, add potassium carbonate (0.5g, 3.4mmol), benzoyl chloride (0.24g, 1.7 mmol), stirred at room temperature for 12h. After the reaction is complete, remove the solvent, add water, extract with ethyl acetate, wash the organic phase with saturated brine, and dry over anhydrous sodium sulfate. Crude column chromatography (V 乙酸乙酯 :V 石油醚 =1:1) to obtain 0.39 g of a yellow solid with a yield of 57% and a melting point of 178-179°C. 1 H NMR (400MHz, DMSO-d 6 )δ8.74(d, J=8.4Hz, 1H, Ar-H), 8.53(s, 1H, Ar-H), 8.31-8.21(m, 2H, Ar-H), 7.91(d, J=7.6 Hz, 2H, Ar-H), 7.82(t, J=7.2Hz, 1H, Ar-H), 7.71(t, J=7.6Hz, 2H, Ar-H), 7.64(d, J=8.0 Hz, 1H, Ar-H), 7.18(d, J=5.2Hz, 1H, Ar-H), 6.78(s, 2H, NH2 ). 13 C NMR (100MHz, DMSO-d 6 ) δ168.9, 164.0, 160.4, 158.8, 137.6, 133.6, 133.2, 130.3, 130.0, 129.4, 127.5, 125.1, 119.3, 11...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com