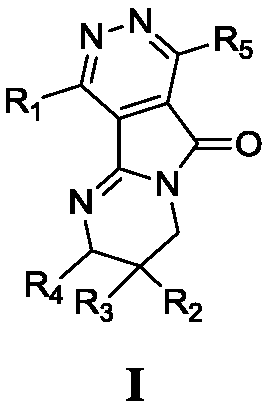
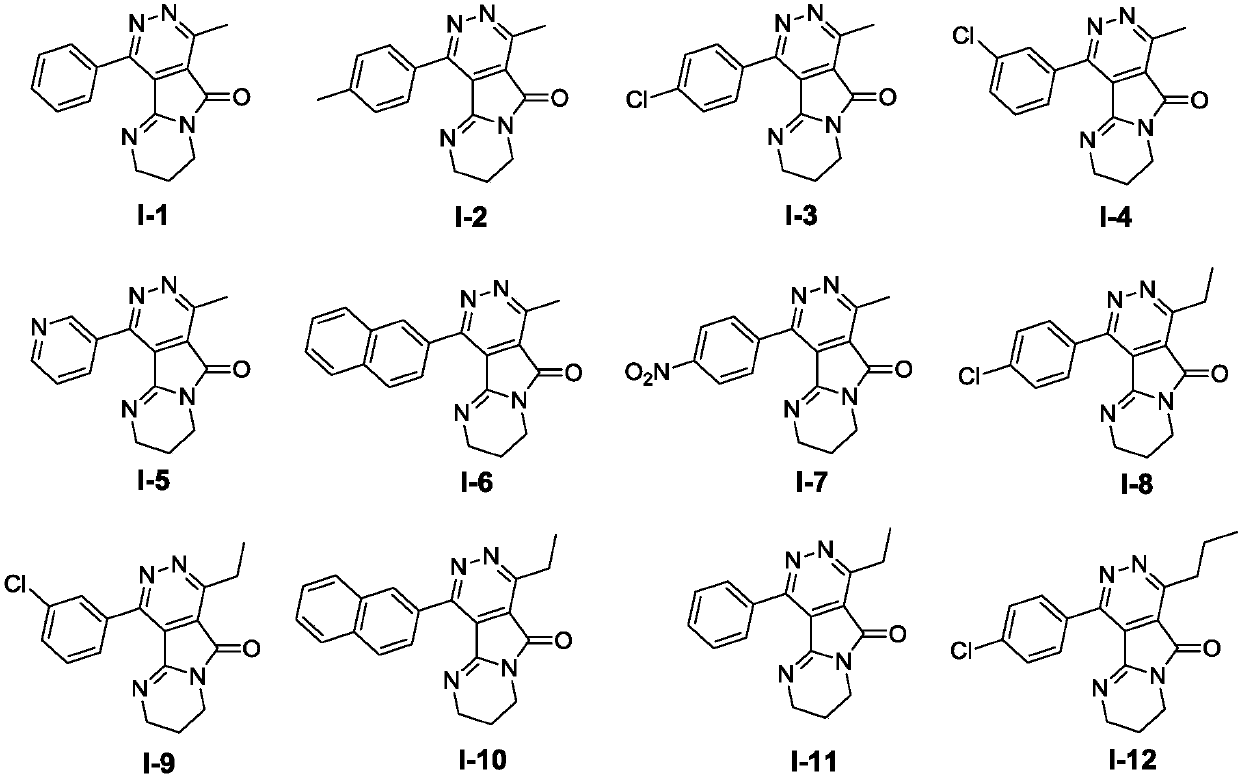
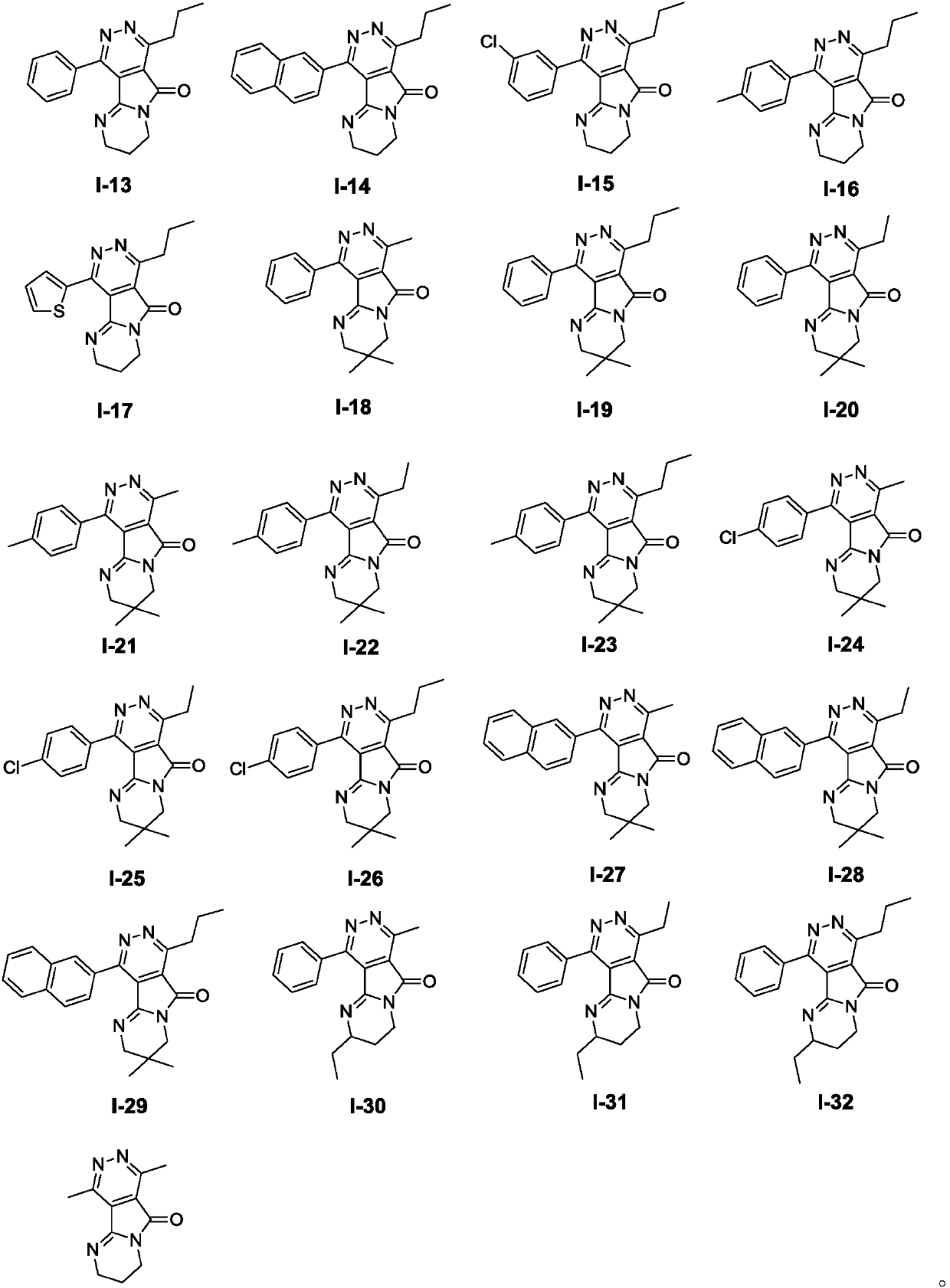
Pyrimido-pyrrolopyridazine derivative as well as intermediate, preparation method, pharmaceutical composition and application
A technology of pyridazine derivatives and pyrimidines, which is applied in the field of pyrimidopyrrolopyridazine derivatives, and can solve the problems of long synthetic route and low total yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Preparation of Compound II-1
[0088] Refer to Huang, Z.-T.; Wang, M.-X., Synthesis 1992, 12, 1273-1276 and Li, Z.-J.; Smith, C.D., Synthetic Communications 2001, 31, 527-533 for specific synthesis The route looks like this:
[0089]
[0090] Dissolve acetophenone (10.0mmol) in THF (50ml) under ice-cooling, add NaH (20.0mmol) and stir for half an hour, then CS 2 (10.0mmol) was added dropwise to the above reaction solution, kept in the ice bath and continued to stir for two hours, and finally MeI (20.0mmol) was dropped into the reaction solution, kept in the ice bath for half an hour, then the temperature was slowly raised to room temperature, and stirred overnight. The reaction was evaporated to dryness under reduced pressure and diluted with EtOAc (100 mL). The organic phase was washed with water (50mL) and saturated brine (50mL) successively, washed with Na 2 SO 4 After drying, the solution was evaporated to dryness under reduced pressure and used directly in t...
Embodiment 2
[0102] Example 2: Preparation of pyrimidopyrrolopyridazine derivative I-1
[0103]
[0104] in CH 3 CN (5ml) was added HKAs (0.2mmol) shown in formula II-1 and DDs (0.2mmol) shown in formula III-1, stirred at room temperature, TLC (thin layer chromatography using silica gel GF254) followed the reaction, Until HKAs and DDs are completely consumed, the compound represented by formula IV-1 is obtained, and the compound is directly subjected to subsequent reactions without isolation.
[0105] Add CuCl to the reactant 2 (0.02 mmol), the resulting mixture was stirred at 50° C. until the compound represented by formula IV-1 was completely converted into product I-1 (monitored by thin-layer chromatography using silica gel GF254). The mixture was cooled to room temperature and diluted with EtOAc (25 mL). The organic phase was washed with saturated NH 4 Cl solution (20mL) and water (20mL) washed with Na 2 SO 4 After drying, the solution was evaporated to dryness on a rotary eva...
Embodiment 3
[0108] Example 3: Preparation of pyrimidopyrrolopyridazine derivatives I-2
[0109]
[0110] The preparation process of pyrimidopyrrolopyridazine derivative I-2 is similar to the preparation process of I-1, and the types of HKAs and DDs are changed to finally obtain yellow solid I-2, melting point: 174.6-175.3 ° C, yield: 65% ,
[0111]
[0112] 1 H NMR (500MHz, CDCl 3 )δ7.96(d, J=8.2Hz, 2H), 7.30(d, J=7.9Hz, 2H), 3.83(q, J=5.6Hz, 4H), 3.11(s, 3H), 2.46–2.41( m,3H),1.97-1.92(m,2H); 13 C NMR (126MHz, CDCl 3 )δ165.21, 155.47, 154.52, 148.65, 140.37, 131.38, 130.48, 128.60, 128.33, 126.31, 47.15, 37.73, 21.52, 19.81, 18.60; HRMS (TOF ES + ):C 17 h 17 N 4 O[(M+H) + ] The predicted value is 293.1397, and the measured value is 293.1398.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com