Method for detecting active ingredients of salviae miltiorrhizae and products
A technology of active ingredients and detection methods, which is applied in the field of quality detection of Chinese medicinal materials and their preparations, can solve the problems of single detection index, unfavorable quality control of Zidanshen medicinal materials and Zidan Huoxue series preparations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
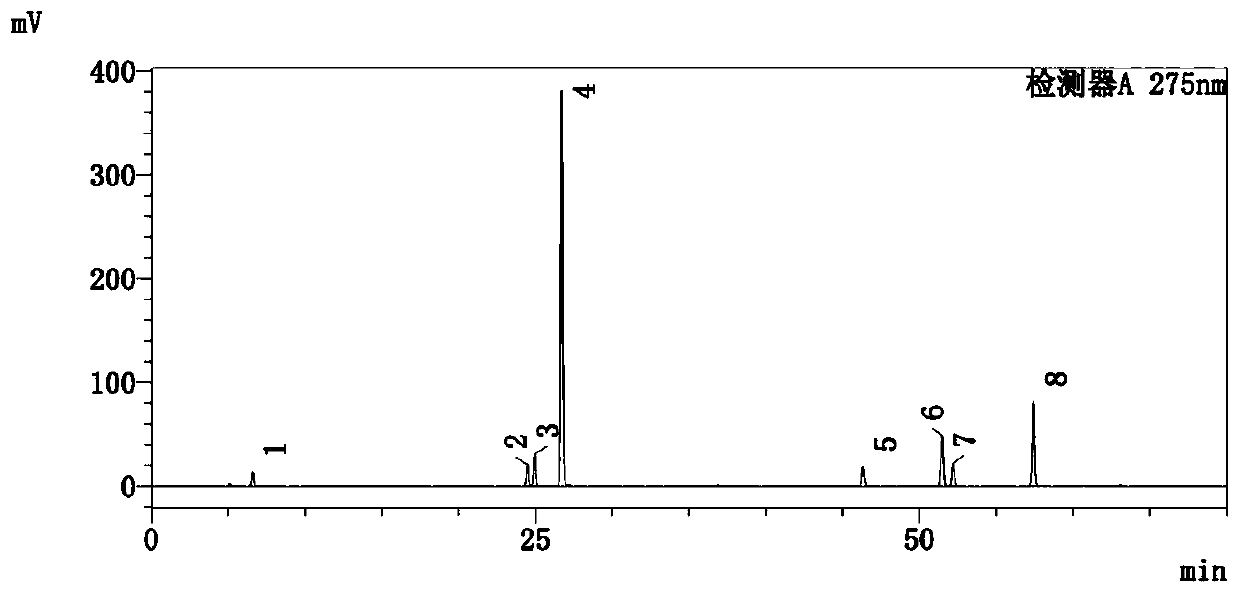
[0098] This example is aimed at the detection method of the active ingredients of the purple salvia miltiorrhiza produced in Chuxiong, Yunnan.
[0099] This example is based on the HPLC determination of the contents of danshenin sodium, rosmarinic acid, shikonic acid, salvianolic acid B, dihydrotanshinone I, cryptotanshinone, tanshinone I, and tanshinone IIA in purple salvia miltiorrhiza produced in Yunnan Chuxiong, including the following steps:
[0100] Step (1), preparation of reference substance solution:
[0101] Accurately weigh the appropriate amount of Danshensu sodium, rosmarinic acid, shikonic acid, salvianolic acid B, dihydrotanshinone Ⅰ, cryptotanshinone, tanshinone Ⅰ, tanshinone ⅡA reference substance, and add methanol to prepare 1 mL of danshensu sodium containing 16.48 μg, Reference substance solution of 12.32 μg of rosmarinic acid, 22.72 μg of shikonic acid, 404 μg of salvianolic acid B, 6.384 μg of dihydrotanshinone I, 14.08 μg of cryptotanshinone, 6.656 μg of...
Embodiment 2
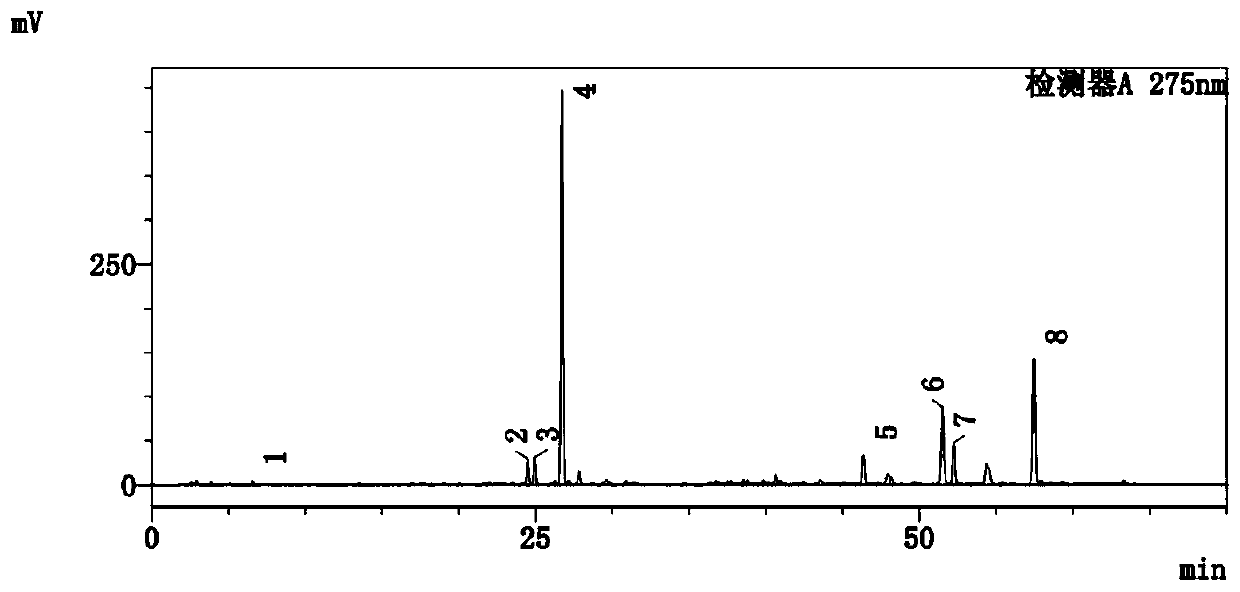
[0113] This example is aimed at the detection method of the active ingredients of wild purple salvia miltiorrhiza.
[0114] In this example, based on HPLC, the content of danshenin sodium, rosmarinic acid, shikonian acid, salvianolic acid B, dihydrotanshinone I, cryptotanshinone, tanshinone I, and tanshinone IIA in wild purple salvia miltiorrhiza is determined, including the following steps:
[0115] Step (1), preparation of reference substance solution:
[0116] Accurately weigh the appropriate amount of Danshensu sodium, rosmarinic acid, shikonic acid, salvianolic acid B, dihydrotanshinone Ⅰ, cryptotanshinone, tanshinone Ⅰ, tanshinone ⅡA reference substance, and add methanol to prepare 4.120 μg of danshensu sodium per 1 mL, Reference substance solution of 3.080 μg of rosmarinic acid, 5.680 μg of shikonic acid, 101 μg of salvianolic acid B, 1.596 μg of dihydrotanshinone I, 3.520 μg of cryptotanshinone, 1.640 μg of tanshinone IIA and 4.200 μg of tanshinone IIA. Instantly.
...
Embodiment 3
[0129] This embodiment is aimed at the detection method of the active ingredients in the medicinal material of Danshen.
[0130] This example is based on the HPLC determination of the contents of danshenin sodium, rosmarinic acid, shikonic acid, salvianolic acid B, dihydrotanshinone I, cryptotanshinone, tanshinone I, and tanshinone IIA in Danshen medicinal materials in various provinces, including the following steps:
[0131] Step (1), preparation of reference substance solution:
[0132] Precisely weigh the appropriate amount of Danshensu Sodium, Rosmarinic Acid, Shikoninic Acid, Salvianolic Acid B, Dihydrotanshinone Ⅰ, Cryptotanshinone, Tanshinone Ⅰ, Tanshinone ⅡA reference substance, add methanol to prepare 41.20 μg of Danshensu Sodium per 1 mL, A solution of 30.80 μg of rosmarinic acid, 56.80 μg of shikonic acid, 1010 μg of salvianolic acid B, 15.96 μg of dihydrotanshinone I, 35.20 μg of cryptotanshinone, 16.40 μg of tanshinone I, and 42.00 μg of tanshinone IIA was obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com