Preparation method and application of a macrophage tracking fluorescent probe
A technology of macrophages and fluorescent probes, applied in the field of preparation of dextran nanoprobes, can solve the problems of renal metabolic clearance, high cost, difficult purification, etc., achieve high biological safety, simple preparation method, and wide application foreground effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
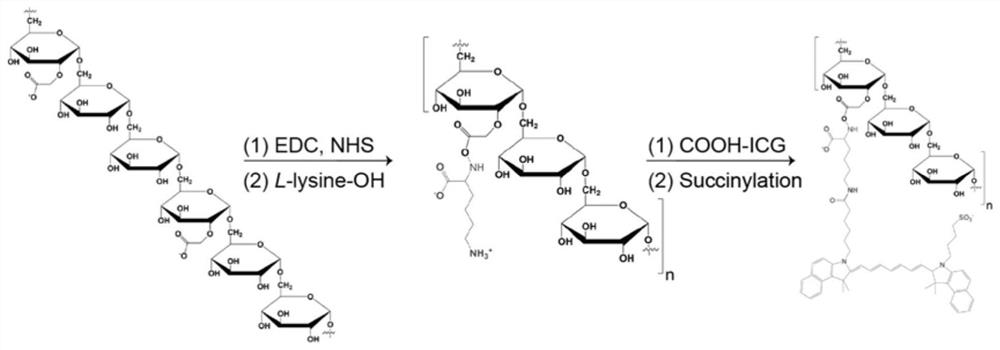
[0033] A method for preparing a macrophage tracking fluorescent probe, comprising the following steps:
[0034] S110, placing carboxymethylated dextran, one of NHS and sulfo-NHS, and an activated carboxyl reagent in a buffer solution, stirring at room temperature to obtain an activated carboxymethylated dextran solution.
[0035]Specifically, in one embodiment, the dextran is at least one carboxymethyl dextran with a molecular weight of 2-40 kD and a carboxyl substitution degree of 2%-10%. The activated carboxyl reagent is at least one of EDC, DCC, CDI and DIC. The buffer can be MES buffer or PBS buffer.
[0036] S120, dissolving the cross-linking agent in the buffer solution, adding it into the activated carboxymethylated dextran solution, stirring and reacting at room temperature to obtain a clear solution.
[0037] Specifically, in one embodiment, the cross-linking agent is lysine.
[0038] S130, adding the clarified solution dropwise to pre-cooled absolute ethanol, cent...
Embodiment 1
[0057] 1. Accurately weigh 0.55g of carboxymethylated dextran, add 2.4g of EDC and 0.4572g of NHS, dissolve in 6.2mL of MES buffer (50mM, pH 6.0-6.5), and react with gentle stirring at room temperature for 10 minutes.
[0058] 2. Accurately weigh 0.4g of L-lysine, dissolve it with 0.7mL MES buffer solution (50mM, pH 6.0-6.5), add it to the activated carboxymethylated dextran solution in step 1, and stir gently at room temperature 5h.
[0059] 3. Add the clear solution obtained in step 2 dropwise to 30mL pre-cooled absolute ethanol, centrifuge (2.5k×g, 3min) to collect the white precipitate, redissolve the obtained white precipitate in water, and pass through 0.22μm filter membrane.
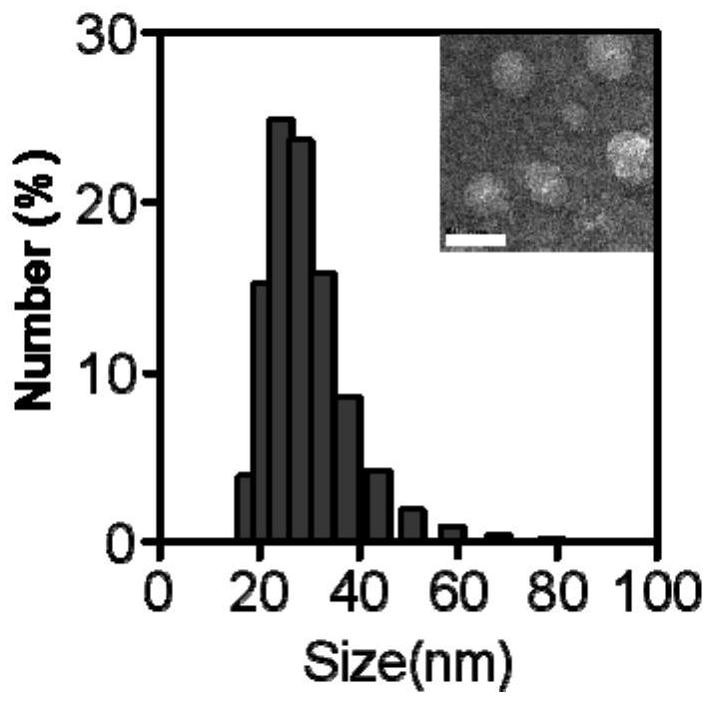
[0060] 4. Use ultrapure water as the dialysis medium to perform room temperature dialysis (3 days) on the solution obtained in step 3 with a 10kD dialysis bag. After dialysis, pass through a 0.22 μm filter membrane, pre-freeze in a -20 degree refrigerator for 2 hours, and transfer to -80 degrees ...
Embodiment 2
[0065] 1. Accurately weigh 0.11g of carboxymethylated dextran, add 0.48g of EDC and 0.09144g of NHS, dissolve in 1.24mL of MES buffer (50mM, pH 6.0-6.5), and stir gently at room temperature for 10 minutes.
[0066] 2. Accurately weigh 0.08g of L-lysine, dissolve it with 0.14mL MES buffer solution (50mM, pH 6.0-6.5), add it to the activated carboxymethylated dextran solution in step 1, and stir gently at room temperature 5h.
[0067] 3. Add the clear solution obtained in step 2 dropwise to 6mL pre-cooled absolute ethanol, centrifuge (2.5k×g, 3min) to collect the white precipitate, redissolve the obtained white precipitate in water, and pass through 0.22μm filter membrane.
[0068] 4. Use ultrapure water as the dialysis medium to perform room temperature dialysis (3 days) on the solution obtained in step 3 with a 10kD dialysis bag. After dialysis, pass through a 0.22 μm filter membrane, pre-freeze in a -20 degree refrigerator for 2 hours, and transfer to -80 degrees Refrigerat...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com