N-substituted aromatic ring-2-aminopyrimidine compounds and their uses
A technology of aminopyrimidine and compound, which is applied in the field of application of N-substituted aromatic ring-2-aminopyrimidine compounds in the preparation of antitumor drugs, can solve the problems of reducing treatment sensitivity, cell cycle arrest, etc., and achieve good oral administration effect, good protein inhibitory activity, good therapeutic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
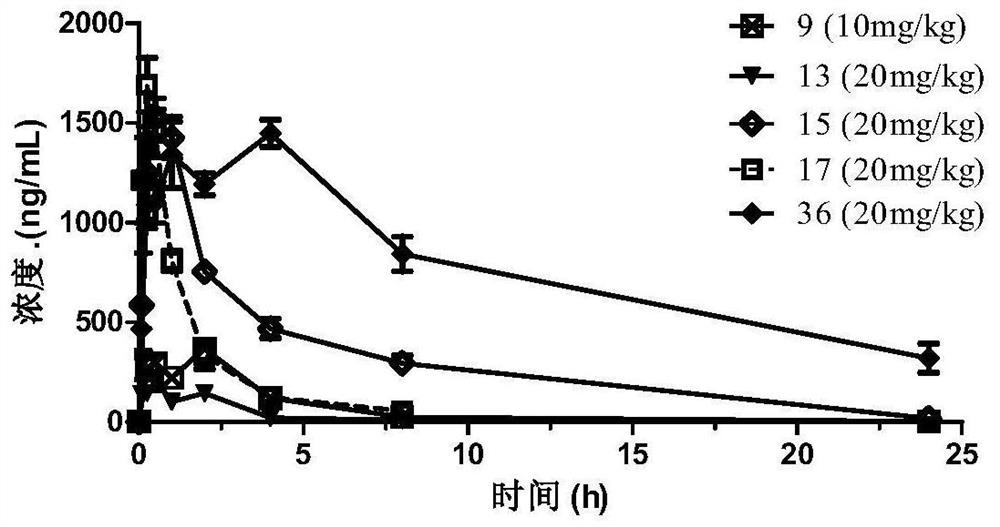
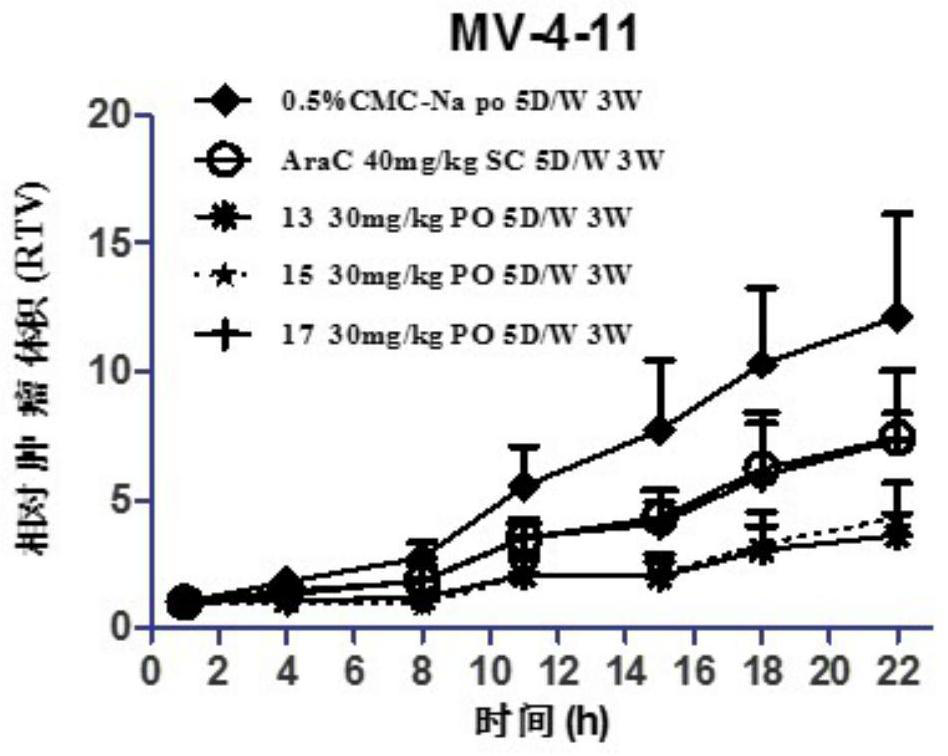
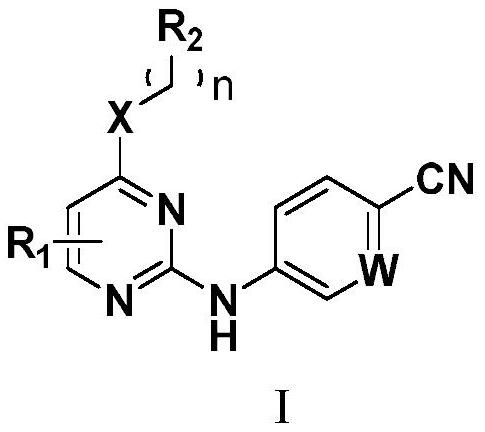
Image
Examples
preparation Embodiment 1
[0125] Preparation Example 1 5-((4-((piperidin-2-ylmethyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-2-cyanopyridine (Compound 1) synthesis
[0126]
[0127] Step 1. Synthesis of 5-trifluoromethyl-4 chloro-2-aminopyrimidine (Intermediate 1-2)
[0128]
[0129] 2,4-Dichloro-5-trifluoromethylpyrimidine (5.2 g, 24.07 mmol) was dissolved in ammonia-saturated ethanol (25 ml), stirred at room temperature for 2 h, and the solvent was recovered under reduced pressure to obtain a residue, which was filtered through a silica gel column Analytical purification, using PE:EA (5:1) as eluent, gave 1-2 (2.3 g, 11.67 mmol) as a white solid, yield: 48.5%. 1 H NMR (500MHz, CDCl 3 ) δ8.57(s, 1H), 7.98(s, 2H). ESI-MS: m / z=198[M+H] + .
[0130] Step 2.N 4 Synthesis of -(N-tert-butoxycarbonylpiperidin-2-ylmethyl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (Intermediate 1-3)
[0131]
[0132] Under ice bath conditions, take intermediate 1-2 (197.0 mg, 1.0 mmol), 1-Boc-2-aminometh...
preparation Embodiment 2
[0136] Preparation Example 2 5-((4-((piperidin-3-ylmethyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-2-cyanopyridine (Compound 2)
[0137]
[0138] Step 1.N 4 Synthesis of -(N-tert-butoxycarbonylpiperidin-3-ylmethyl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (Intermediate 1-4)
[0139]
[0140] The synthesis steps refer to step 2 of Example 1. Intermediate 1-4 was synthesized by substituting 1-Boc-3-aminomethylpiperidine for 1-Boc-2-aminomethylpiperidine. Yield: 70%. 1 H NMR (500MHz, CDCl 3 )δ8.08(s, 1H), 5.44(s, 1H), 5.10(s, 2H), 3.85(m, 2H), 3.40(m, 2H), 3.01(m, 1H), 2.88–2.73(m ,1H),1.91–1.81(m,2H),1.77(m,2H),1.68
[0141] (m,1H),1.47(s,9H).ESI-MS: m / z=376[M+H] + .
[0142] Step 2. Synthesis of 5-((4-((piperidin-3-ylmethyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-2-cyanopyridine (compound 2)
[0143]
[0144] Synthesis steps Refer to Example 1, Step 3 to synthesize Compound 2. Yield: 65%. 1 H NMR (500MHz, DMSO) δ 10.30 (s, 1H), 9.07 (d, J=2.5H...
preparation Embodiment 3
[0146] Preparation Example 3 (R)-5-((4-((morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-2-cyanopyridine (Compound 3)
[0147]
[0148] Step 1. (R)-N 4 Synthesis of -(N-tert-butoxycarbonylmorpholin-2-ylmethyl)-5-(trifluoromethyl)pyrimidine-2,4-diamine (Intermediate 1-5)
[0149]
[0150] Synthesis steps refer to Example 1, Step 2. Intermediate 1-5 was synthesized by substituting (S)-4-N-Boc-2-aminomethylmorpholine for 1-Boc-2-aminomethylpiperidine. Yield: 65%. 1 H NMR (500MHz, CDCl 3 )δ8.09(s,1H),5.50(s,1H),5.10(s,2H),4.13–3.67(m,4H),3.66–3.49(m,2H),3.47–3.37(m,1H) ,2.96(s,1H),2.69(s,1H),1.49(s,9H).ESI-MS:m / z=378[M+H] + .
[0151] Step 2. (R)-5-((4-((morpholin-2-ylmethyl)amino)-5-(trifluoromethyl)pyrimidin-2-yl)amino)-2-cyanopyridine ( Compound 3) Synthesis
[0152]
[0153] Synthesis steps Refer to Example 1, Step 3 to synthesize compound 3. Yield: 70%. 1 H NMR (500MHz, DMSO) δ 10.35(s, 1H), 9.04(d, J=2.0Hz, 1H), 8.50–8.42(m, 1H), 8.31...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com