Compound bone peptide medicine composition oral preparation and preparation method therefor
A technology of compound osteopeptide and composition, which is applied in the direction of drug combination, non-active ingredients of polymer compounds, antipyretics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
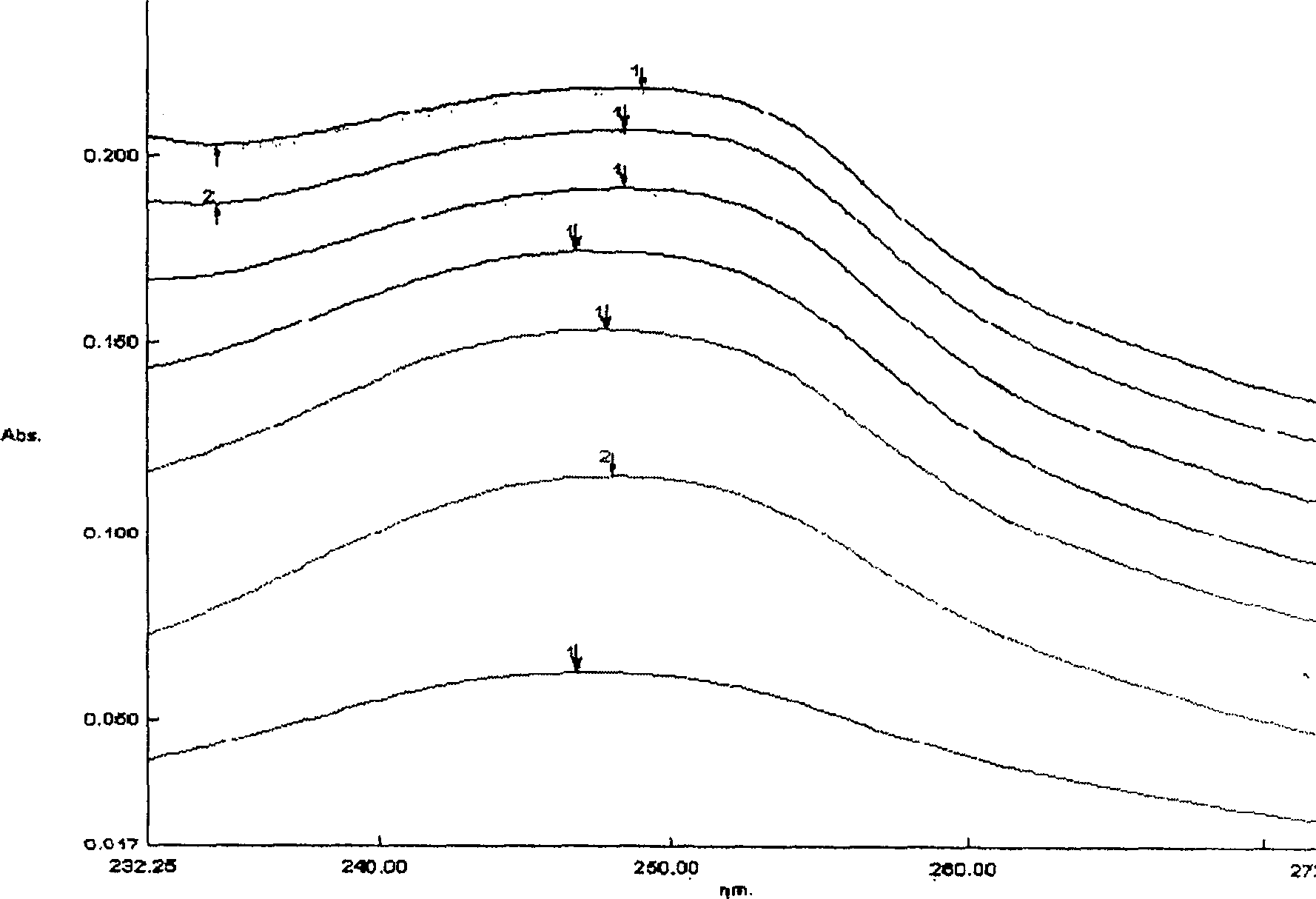
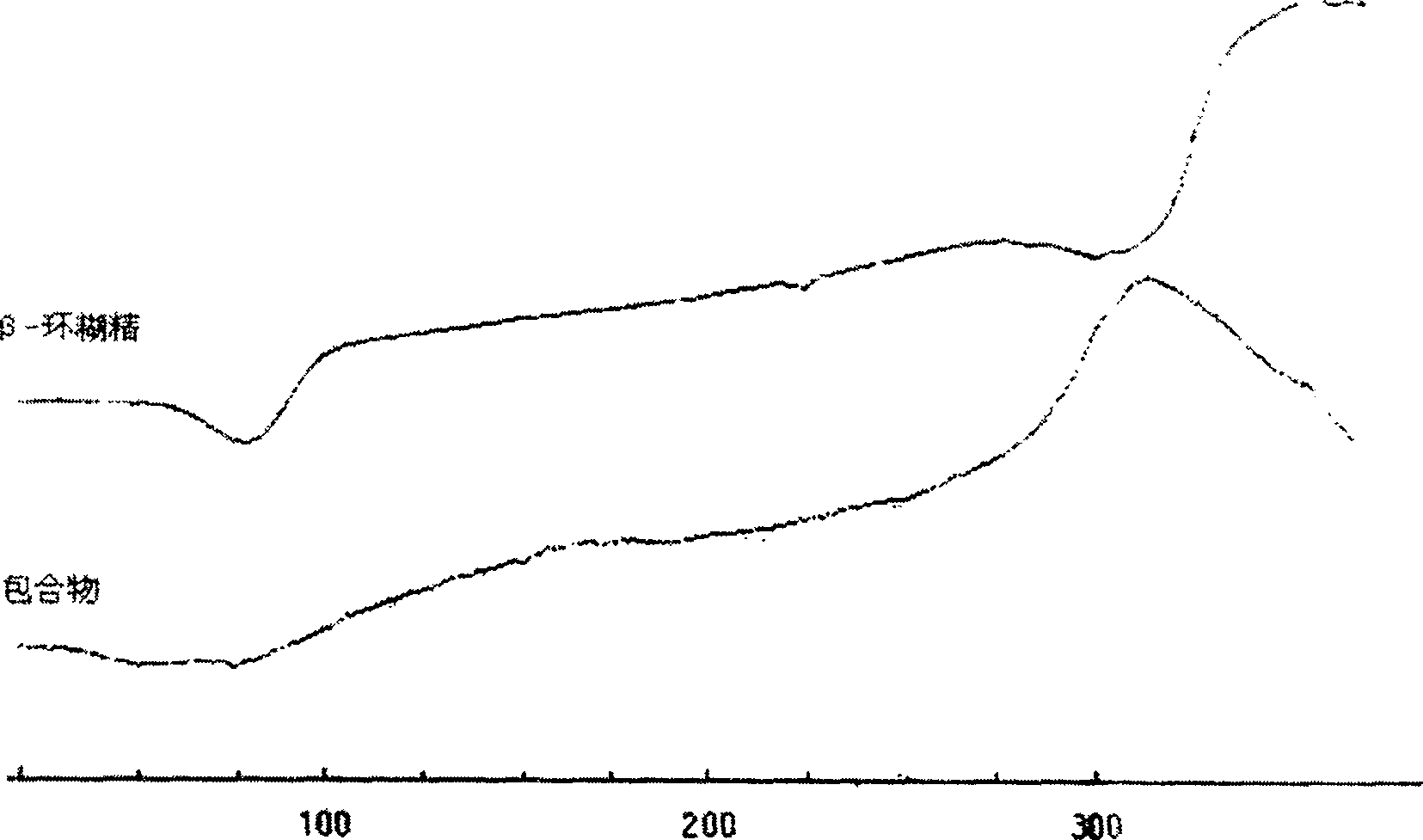
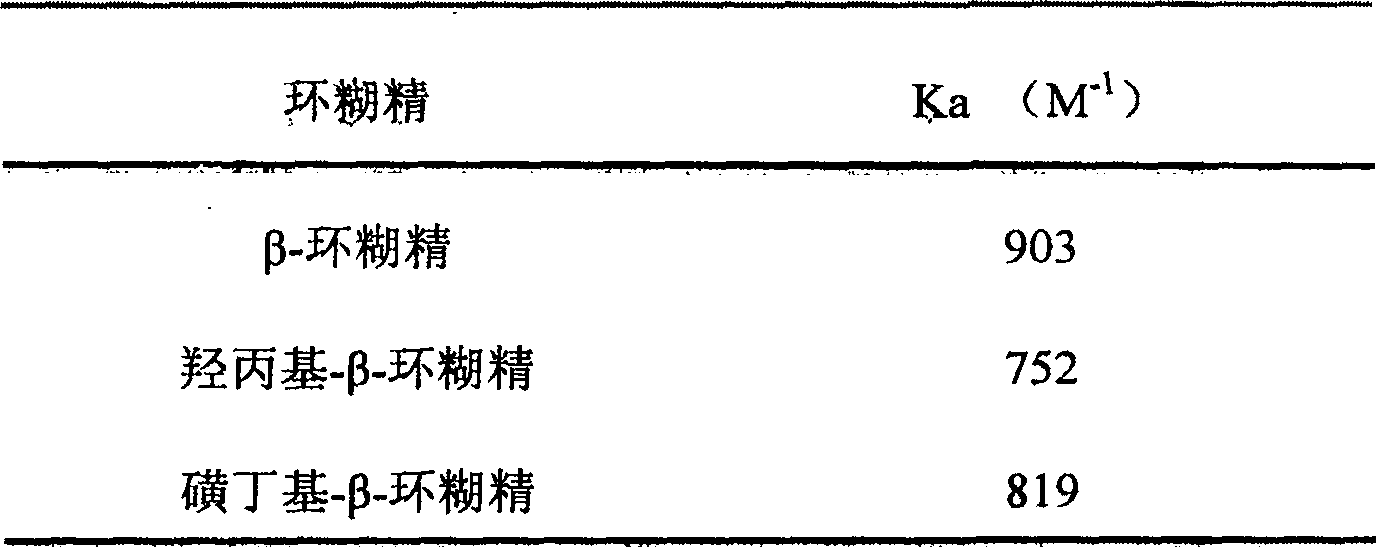
[0092] Example 1, oral compound osteopeptide pharmaceutical composition: mix 1100 grams of β-cyclodextrin with 6600 ml of pure water to form a suspension, add 100 grams of paste compound osteopeptide extract under stirring, mix well and then stir After 1 hour, the system was uniformly in the form of a paste, and dried under reduced pressure at 60°C to obtain 1117 g of light yellow solid clathrates.
[0093] The obtained solid clathrate is passed through a 100-mesh sieve for later use, mixed with 100 grams of microcrystalline cellulose, 150 grams of starch, 200 grams of carboxymethyl starch sodium, 20 grams of talcum powder, and 26 grams of stearic acid and then taking 2 / 3 of the medicine Mix excipients and solid clathrates, mix thoroughly, moisten with 70% ethanol to make soft material, pass through 14 mesh sieve to granulate, dry at 50°C, add another 1 / 3 amount of pharmaceutical excipients after sizing, and mix thoroughly Afterwards, the content of the compound bone peptide e...
Embodiment 2
[0094] Example 2: Mix 900 grams of β-cyclodextrin with 2500 milliliters of pure water to form a suspension, add 300 grams of pasty compound bone peptide extract under stirring, mix well and then stir for an hour, the system is evenly paste , and dried under reduced pressure at 60°C to obtain 1136 g of light yellow solid clathrate.
[0095] The obtained solid clathrate is passed through a 100-mesh sieve for later use, mixed with 100 grams of microcrystalline cellulose, 100 grams of starch, 250 grams of carboxymethyl starch sodium, 20 grams of talcum powder, and 26 grams of stearic acid and then taking 2 / 3 of the medicine Mix excipients and solid clathrates, mix thoroughly, moisten with 70% ethanol to make soft material, pass through 14 mesh sieve to granulate, dry at 50°C, add another 1 / 3 amount of pharmaceutical excipients after sizing, and mix thoroughly After the determination of the content, the compound bone peptide inclusion tablet was obtained by tableting.
Embodiment 3
[0096] Example 3: Mix 960 grams of β-cyclodextrin with 2000 milliliters of pure water to form a suspension, add 240 grams of pasty compound bone peptide extract under stirring, mix well and then stir for an hour, the system is evenly paste , and dried under reduced pressure at 60°C to obtain 1126 g of light yellow solid clathrate.
[0097] The obtained solid clathrate is passed through a 100-mesh sieve for later use, mixed with 100 grams of microcrystalline cellulose, 100 grams of starch, 250 grams of carboxymethyl starch sodium, 20 grams of talcum powder, and 26 grams of stearic acid and then taking 2 / 3 of the medicine Mix excipients and solid clathrates, mix thoroughly, moisten with 70% ethanol to make soft material, pass through 14 mesh sieve to granulate, dry at 50°C, add another 1 / 3 amount of pharmaceutical excipients after sizing, and mix thoroughly After the determination of the content, the compound bone peptide inclusion tablet was obtained by tableting.
PUM
| Property | Measurement | Unit |
|---|---|---|
| control rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com