Purple active dye based on triazo-chromogenic body, and preparation and application of same
A technology of reactive dyes and trisazo, which is applied in the field of reactive dyes and their preparation and application, can solve the problems of insufficient lifting performance and color fastness, poor compatibility, etc., and achieve novel shades, good strength performance and color fastness, The effect of lifting force performance and color fastness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
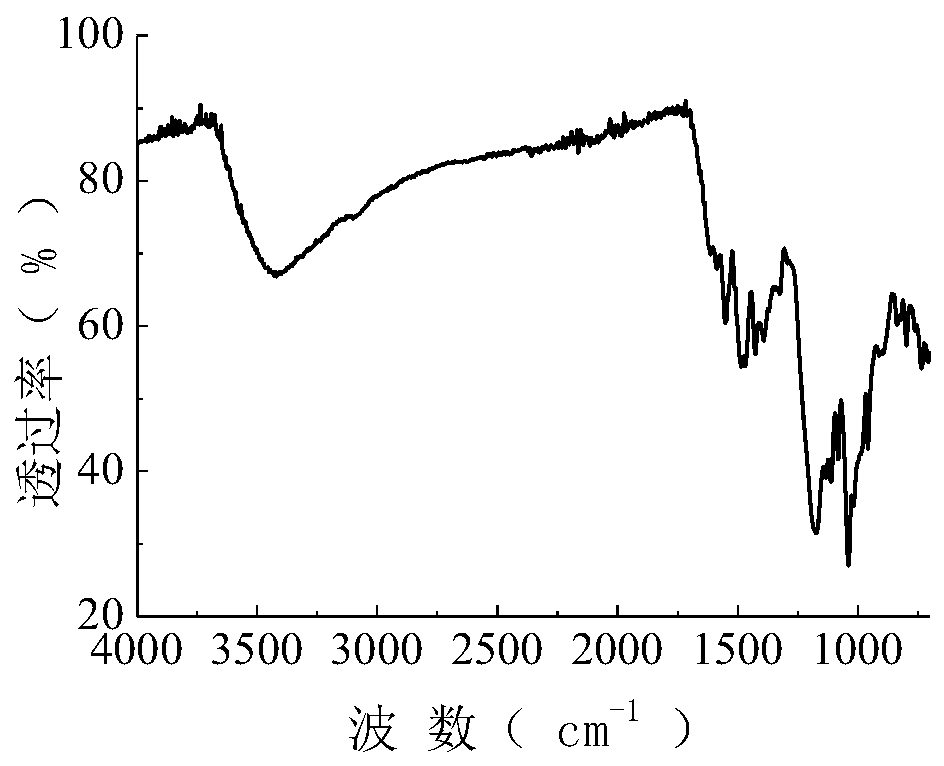
[0048] The synthetic technique of the purple reactive dye with following structure:
[0049]
[0050](1) Preparation of a condensate: 18.8 g (0.102 mol) of cyanuric chloride was configured into a suspension with a mass fraction of 20%, and it was beaten in an ice-water bath at 0-2°C for 0.6 h. Accurately weigh 34.1g (0.100mol) H acid, configure it into an aqueous solution with a mass fraction of 20%, adjust its pH value to 6.0 with 10% NaOH, and add it dropwise to the beating at 0-5°C In the cyanuric chloride, keep the temperature at 3~5℃ after the dropwise addition, and use 15% Na 2 CO 3 Adjust the pH value to 4.5, react for 4 hours, and detect the end point of the shrinkage reaction by thin-layer chromatography (developing solvent: n-butanol: ethanol: ammonia water = 6:2:3).
[0051] (2) Preparation of diazonium salt of anthranilsulfonic acid: Accurately weigh 17.3g (0.098mol) of anthranilsulfonic acid, configure it into an aqueous solution with a mass fraction of 20%, ...
Embodiment 2
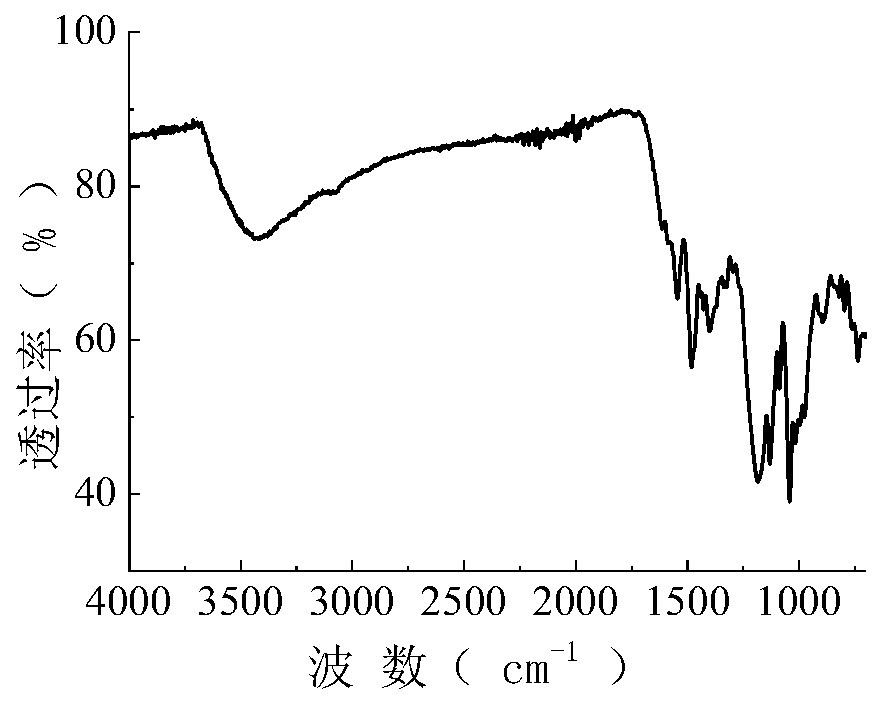
[0059] The synthetic method of present embodiment active dye is the same as embodiment 1, and raw material 17.3g (0.098mol) anthranilic acid is changed into 26.9g (0.098mol) aniline-2,5-disulfonic acid in embodiment 1, and all the other are all mixed with Embodiment 1 is identical, obtains the following purple reactive dyes of structure, and productive rate is 75.1% (according to H acid charging amount calculation). The resulting dye was purified by multiple recrystallizations from a mixture of ethanol and water (2:1), and its infrared spectrum was shown in figure 2 , the data are as follows: 3442.2, 1550.2, 1476.0, 1382.3, 1326.9, 1183.7, 1301.5, 1182.9, 1126.8, 1033.3, 1007.7, 902.0, 802.1cm -1 .
[0060]
Embodiment 3
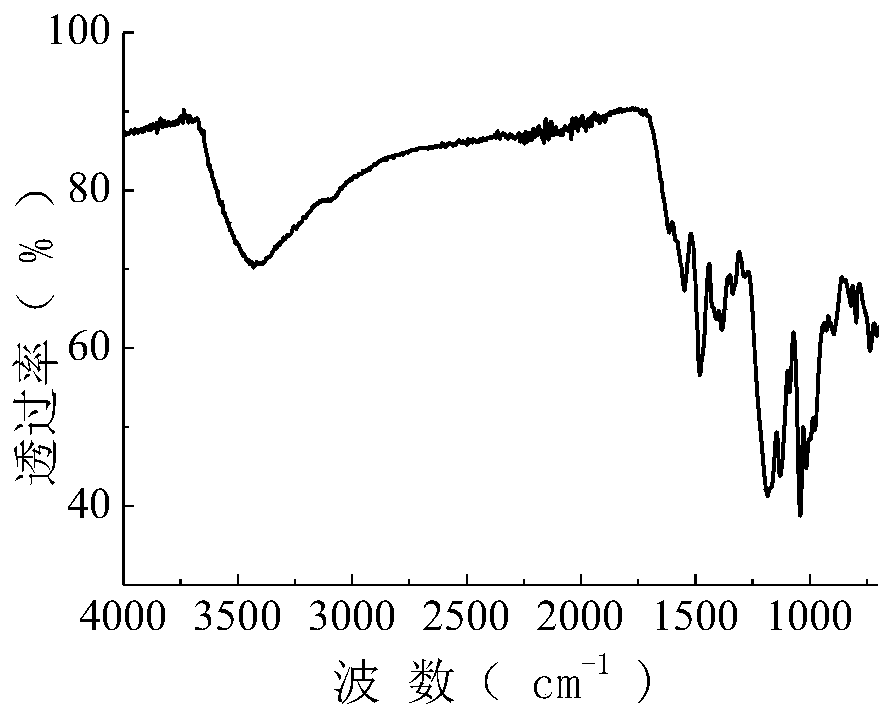
[0062] The synthetic method of present embodiment active dye is the same as embodiment 1, and the raw material 17.3g (0.098mol) anthranilic acid is changed into 27.5g (0.098mol) 4-ethyl sulfate sulfone aniline in embodiment 1, and all the other are all the same as the implementation Example 1 is identical, obtains the following purple reactive dyes of structure, and productive rate is 76.9% (according to H acid charging amount calculation). The resulting dye was purified by multiple recrystallizations from a mixture of ethanol and water (2:1), and its infrared spectrum was shown in image 3 , the data are as follows: 3423.5, 1544.5, 1475.7, 1419.7, 1382.1, 1326.1, 1175.8, 1075.8, 1032.6, 957.5, 901.7, 795.2cm -1 .
[0063]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com