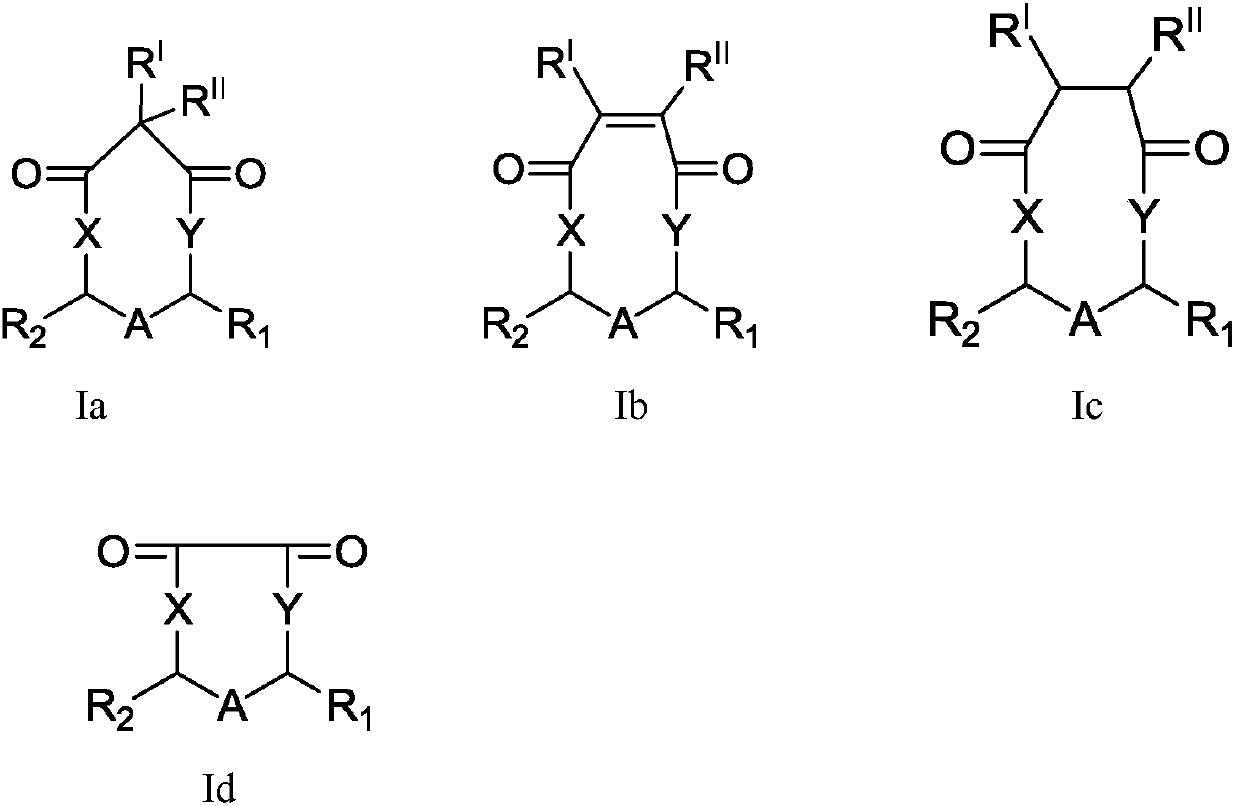
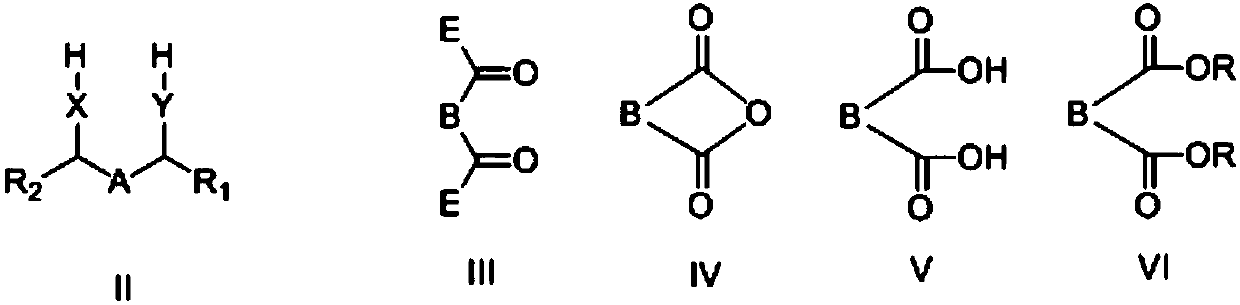
Cyclic compound, preparation method and applications thereof
A kind of technology of heterocyclic group and dioxone ring, which is applied in the field of cyclic compound and its preparation and application, and can solve the problems such as low overall effect of catalyst
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] Example 1 Synthesis of compound 2,4-dimethyl-1,5-dioxonane-6,9-dione
[0072] In a 250 ml three-necked flask, add 2.08 g of 2,4-pentanediol, 100 ml of acetonitrile, 2.15 ml of triethylamine, and 0.35 g of anhydrous K after blowing with nitrogen. 2 CO 3 , Add 3.48 g of diethyl succinate dropwise at room temperature and stir evenly. The reaction was stirred for 2 hours and then heated and refluxed for 8 hours. After concentration under reduced pressure, the product was separated by column chromatography to obtain 1.71 g of a colorless liquid product (yield 46%). 1 H-NMR(δ, ppm, TMS, CDCl 3 ): 4.15~4.13(2H,m,OCH), 2.67~2.65(2H,m,CH 2 ),2.58~2.58(2H,m,CH 2 ),1.88~1.87(1H,m,CH 2 ),1.65~1.64(1H,m,CH 2 ),1.44~1.42(6H,m,CH 3 ); Mass spec, GD-Mass:186.
Embodiment 2
[0073] Example 2 Synthesis of compound 2,3,4-trimethyl-1,5-dioxonane-6,9-dione
[0074] In a 250 ml three-necked flask, add 2.36 g of 3-methyl-2,4-pentanediol, 100 ml of acetonitrile, 2.15 ml of triethylamine and 0.20 g of anhydrous potassium chloride after blowing with nitrogen at room temperature Add 3.06 g of succinyl chloride dropwise and stir evenly. The reaction was stirred for 4 hours and then heated and refluxed for 8 hours. After concentration under reduced pressure, the product was separated by column chromatography to obtain 1.60 g of a colorless liquid product (yield 40%). 1 H-NMR(δ, ppm, TMS, CDCl 3 ): 4.16~4.14(2H,m,OCH), 2.67~2.65(2H,m,CH 2 ),2.60~2.59(2H,m,CH 2 ), 2.84~2.83(1H,m,CH),1.44~1.42(6H,m,CH 3 ),1.05~1.03(3H,d,CH 3 ); mass spectrum, GD-mass spectrum: 200.
Embodiment 3
[0075] Example 3 Synthesis of compound 2,4-diethyl-1,5-dioxonane-6,9-dione
[0076] In a 250 ml three-necked flask, after nitrogen blowing, add 2.64 g of 3,5-heptanediol, 120 ml of acetonitrile, 2.15 ml of triethylamine and 0.20 g of anhydrous potassium chloride, and add 3.06 g of butyl at room temperature. Diacid chloride, stir well. The reaction was stirred for 2 hours and then heated and refluxed for 10 hours. After concentration under reduced pressure, the product was separated by column chromatography to obtain 1.50 g of a colorless liquid product (yield 35%). 1 H-NMR(δ, ppm, TMS, CDCl 3 ): 3.95~3.94(2H,m,OCH),2.66~2.64(2H,m,CH 2 ),2.61~2.59(2H,m,CH 2 ),1.90~1.89(1H,m,CH),1.64~1.63(1H,m,CH 2 ),1.57~1.53(4H,m,CH 2 ),0.97~0.95(6H,m,CH 3 ); Mass spec, GD-Mass: 214.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com