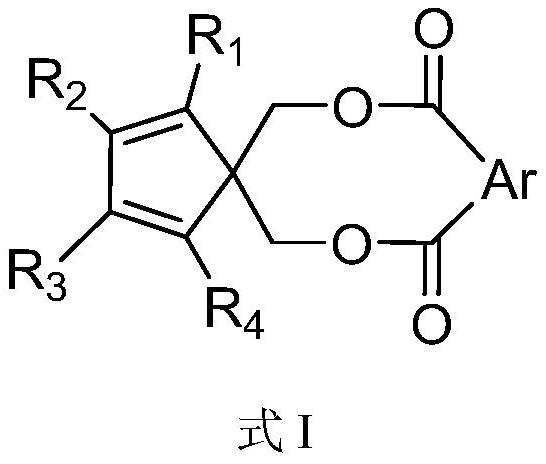
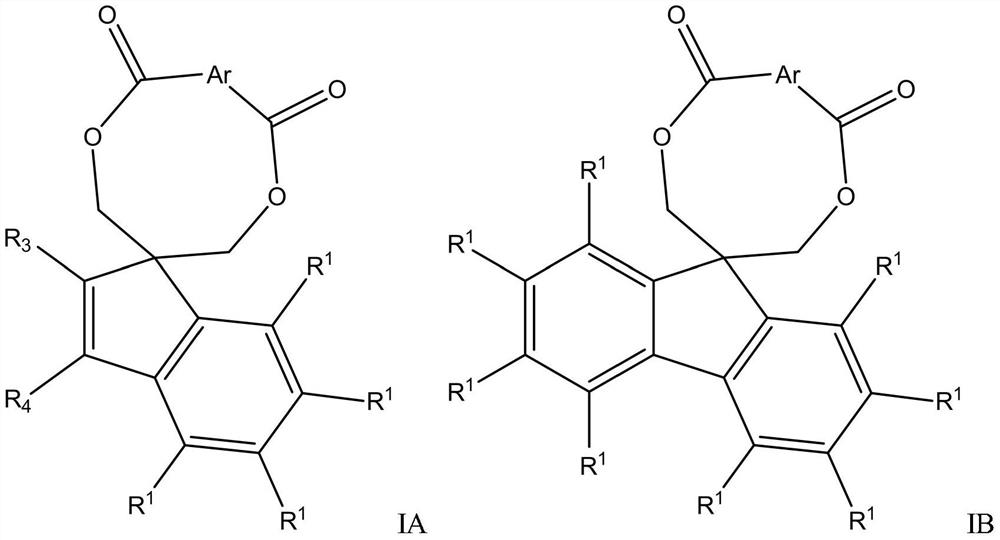
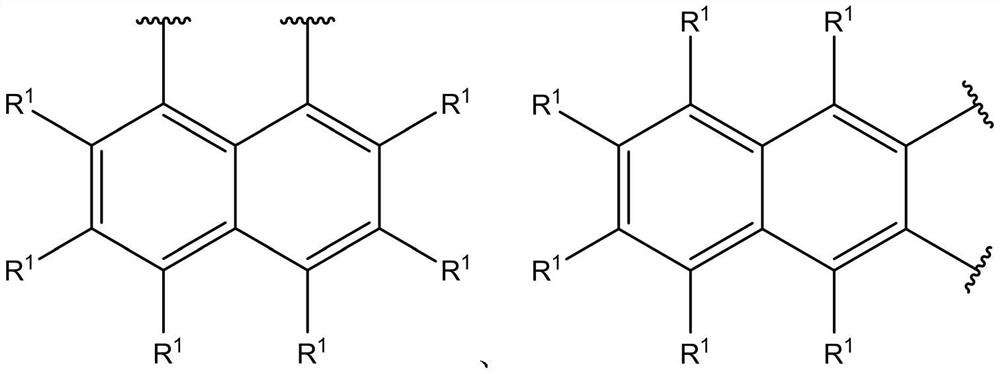
Catalyst component for olefin polymerization, catalyst and olefin polymerization method
A technology for olefin polymerization and catalyst, applied in the field of catalyst components, can solve the problem of not outstanding overall effect of the catalyst, and achieve the effects of adjustable isotactic index, wide molecular weight distribution and good hydrogen modulation sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Example 1 Compound 8,9-benzo[h]-2,6-dioxa-4,9'-fluorenyl-1,7-cyclononanedione (FC-1)
[0068] In a 250 ml three-necked flask, add 4.52 g of 9,9-dimethylolfluorene, 120 ml of acetonitrile, 2.15 ml of triethylamine and 0.22 g of potassium chloride after purging with nitrogen, and add 5.02 g of potassium chloride dropwise at room temperature. gram of phthaloyl chloride, and stir well. Stir the reaction for 4 hours, then raise the temperature and reflux for 8 hours. After concentrating under reduced pressure, recrystallize from a mixed solution of diethyl ether / petroleum ether (1:50) to obtain light yellow crystals, and dry in vacuo to obtain 2.42 g of the product (yield 34%). 1 H-NMR (δ, ppm, TMS, CDCl 3 ):8.10~8.08(2H,m,ArH),7.85~7.83(2H,m,ArH),7.58~7.55(4H,m,ArH),7.37~7.36(2H,m,ArH),7.26~7.24( 2H,m,ArH),4.83~4.81(4H,m,OCH 2 ).
Embodiment 2
[0069] Example 2 Compound 8,9,10-naphtho[1',8'-hi]-2,6-dioxa-4,9'-fluorenyl-1,7-cyclodecanedione (FC-2 )
[0070] In a 250 ml three-neck flask, add 4.52 g of 9,9-dimethylolfluorene, 120 ml of acetonitrile, 2.15 ml of triethylamine and 0.32 g of potassium chloride after purging with nitrogen, and add 5.00 g of potassium chloride dropwise at room temperature. gram of 1,8-naphthalene dicarbonyl chloride, and stir well. After stirring for 4 hours, the temperature was raised to reflux for 10 hours. After concentrating under reduced pressure, recrystallize from a mixed solution of diethyl ether / petroleum ether (1:50) to obtain yellow crystals, and dry in vacuo to obtain 2.43 g of the product (30% yield). 1 H-NMR (δ, ppm, TMS, CDCl 3 ):8.35~8.32(2H,m,ArH),7.98~7.96(2H,m,ArH),7.85~7.83(2H,m,ArH),7.56~7.54(2H,m,ArH),7.47~7.45( 2H,m,ArH),7.38~7.36(2H,m,ArH),7.28~7.26(2H,m,ArH),4.83~4.81(4H,m,OCH 2 ).
Embodiment 3
[0071] Example 3 Compound 8,9,10,11-dibenzo[hj]-2,6-dioxa-4,9'-fluorenylundecacyclo-1,7-dione (FC-3)
[0072] In a 250 ml three-necked flask, add 4.52 g of 9,9-dimethylolfluorene, 130 ml of acetonitrile, 2.15 ml of triethylamine and 0.32 g of potassium chloride after purging with nitrogen, and add 5.54 g of potassium chloride dropwise at room temperature. gram of 1,1′-biphenyl dicarboxylic acid chloride, and stir well. After stirring for 4 hours, the temperature was raised to reflux for 14 hours. After concentrating under reduced pressure, recrystallize from a mixed solution of diethyl ether / petroleum ether (1:50) to obtain yellow crystals, and dry in vacuo to obtain 2.59 g of the product (yield 30%). 1 H-NMR (δ, ppm, TMS, CDCl 3 ):8.05~8.03(2H,m,ArH),7.85~7.83(2H,m,ArH),7.59~7.58(2H,m,ArH),7.55~7.53(4H,m,ArH),7.38~7.36( 2H,m,ArH),7.33~7.31(2H,m,ArH),7.28~7.26(2H,m,ArH),4.84~4.81(4H,m,OCH 2 ).
[0073] (2) Preparation of solid components in the catalyst and propylene poly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com