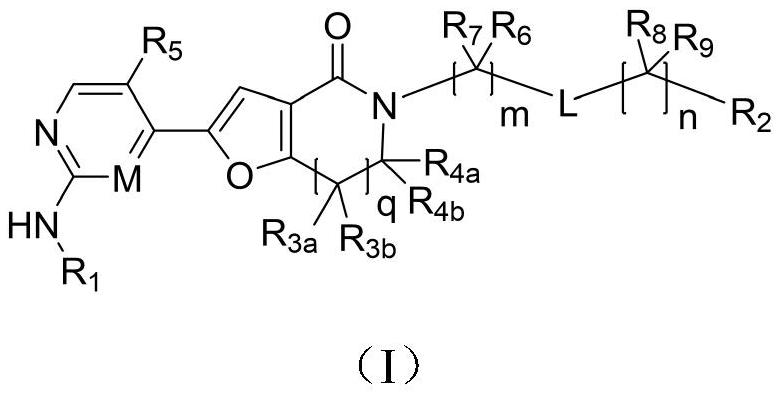
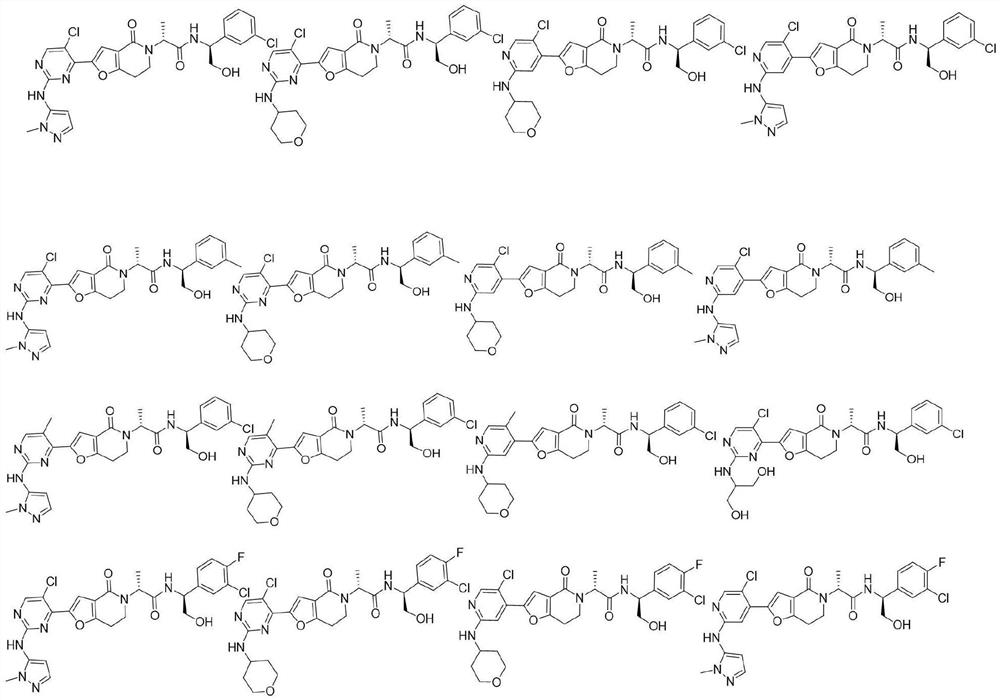
A class of furolactam compounds, preparation method and use
A compound and alkyl technology, applied in the field of medicinal chemistry, can solve the problems of low activity, single structure, easy drug resistance, etc., and achieve the effects of good ERK kinase inhibitory activity, novel structure, and excellent cell proliferation inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0163] Example 1: (R)-N-((S)-1-(3-chlorophenyl)-2-hydroxyethyl)-2-(2-(5-methyl-2-((1-methyl Base-1H-pyrazol-5-yl)amino)pyridin-4-yl)-4-oxo-6,7-dihydrofuro[3,2-c]pyridin-5(4H)-yl)propionamide
[0164]
[0165] The first step: (4-chloro-5-methylpyridin-2-yl) (1-methyl-1H-pyrazol-5-yl) tert-butyl carbamate (5g, 15.5mmol), pinacol Borate (15.8g, 62.1mmol) was dissolved in dimethyl sulfoxide DMSO (50mL), and Pd-XPhos-G2 (1.2g, 1.5mmol), 2-dicyclohexylphosphine-2',4' were added under nitrogen protection , 6'-triisopropylbiphenyl XPhos (1.4g, 3.0mmol) and potassium acetate (3g, 31mmol), heated to 75 degrees overnight. The solution was cooled to room temperature, filtered, and the residue was purified by column chromatography to obtain (2-((tert-butoxycarbonyl)(1-methyl-1H-pyrazol-5-yl)amino)-5-methylpyridine-4 -yl) pinacol borate (2 g, white solid). LC-MS:ESI[M+H] + = 415.3.
[0166] The second step: (2-((tert-butoxycarbonyl)(1-methyl-1H-pyrazol-5-yl)amino)-5-methylpyridin-4-...
Embodiment 2
[0169] Example 2: (R)-N-((6-(dimethylamino)pyridin-2-yl)methyl)-2-(2-(5-methyl-2-((1-methyl- 1H-pyrazol-5-yl)amino)pyridin-4-yl)-4-oxo-6,7-dihydrofuro[3,2-c]pyridin-5(4H)-yl)propionamide
[0170] It was synthesized by the same method as in Example 1, LC-MS: ESI (M+H) 529.4. 1 H-NMR (DMSO-d6, 400MHz) 8.76(s, 1H), 8.33-8.37(m, 1H), 8.04(s, 1H), 7.44(t, J=7.6Hz, 1H), 7.31(d, J =5.6Hz,1H),7.13(s,1H),7.07(s,1H),6.46(t,J=8.4Hz,2H),6.24(s,1H),5.14-5.19(m,1H),4.21 (d,J=6.0Hz,2H),3.63-3.70(m,5H),3.00-3.10(m,2H),2.98(s,6H),2.38(s,3H),1.37(d,J=7.2 Hz, 3H).
Embodiment 3
[0171] Example 3: (R)-N-((6-(dimethylamino)pyridin-2-yl)methyl)-2-(2-(2-((1-methyl-1H-pyrazole- 5-yl)amino)pyridin-4-yl)-4-oxo-6,7-dihydrofuro[3,2-c]pyridin-5(4H)-yl)propionamide
[0172] Synthesized by the same method as in Example 1, LC-MS: ESI (M+H) 515.5. 1 H-NMR (DMSO-d6, 400MHz) 9.52(br s, 1H), 8.63(t, J=5.6Hz, 1H), 8.12(d, J=6.0Hz, 1H), 7.82(t, J=8.0Hz ,1H),7.61(s,1H),7.47(d,J=2.0Hz,1H),7.29(d,J=5.6Hz,1H),7.13(s,1H),6.98(d,J=8.4Hz ,1H),6.67(d,J=7.2Hz,1H),6.34(d,J=2.0Hz,1H),5.07-5.09(m,1H),4.38-4.40(m,2H),3.68-3.71( m, 5H), 3.18 (s, 6H), 3.12 (t, J=7.2Hz, 2H), 1.38 (d, J=7.2Hz, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com