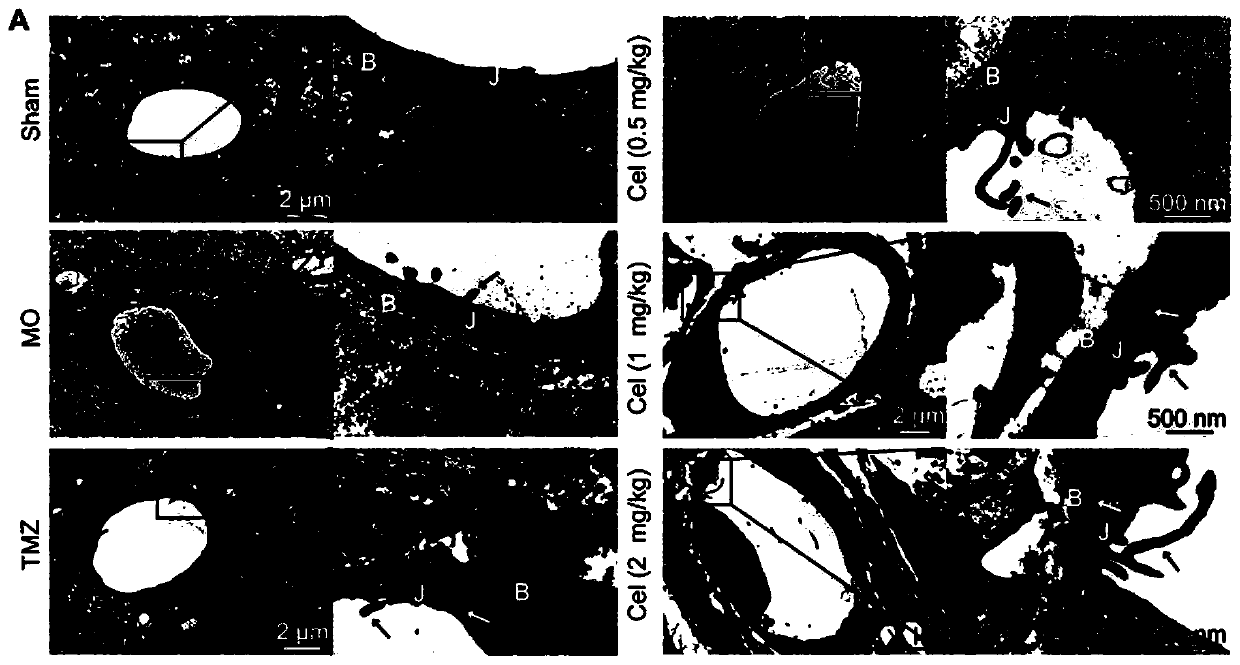
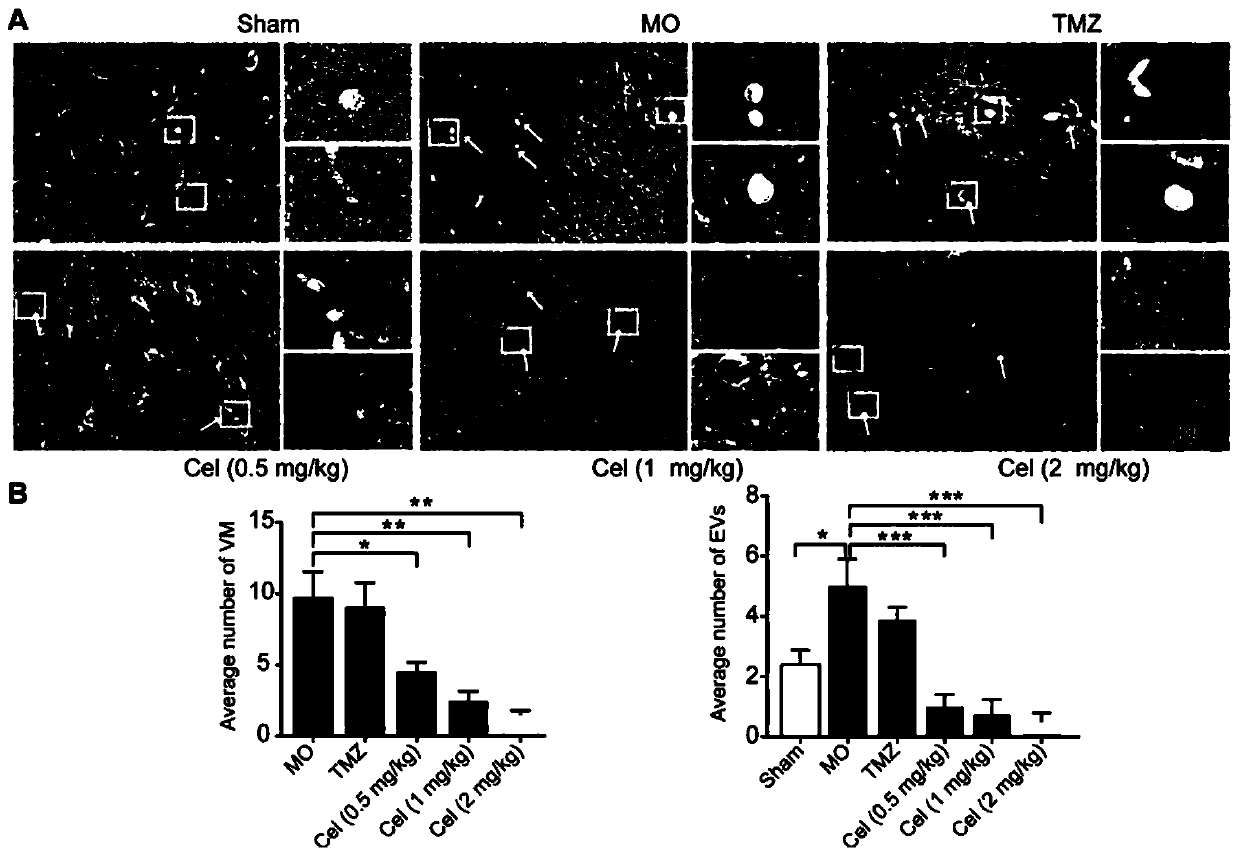
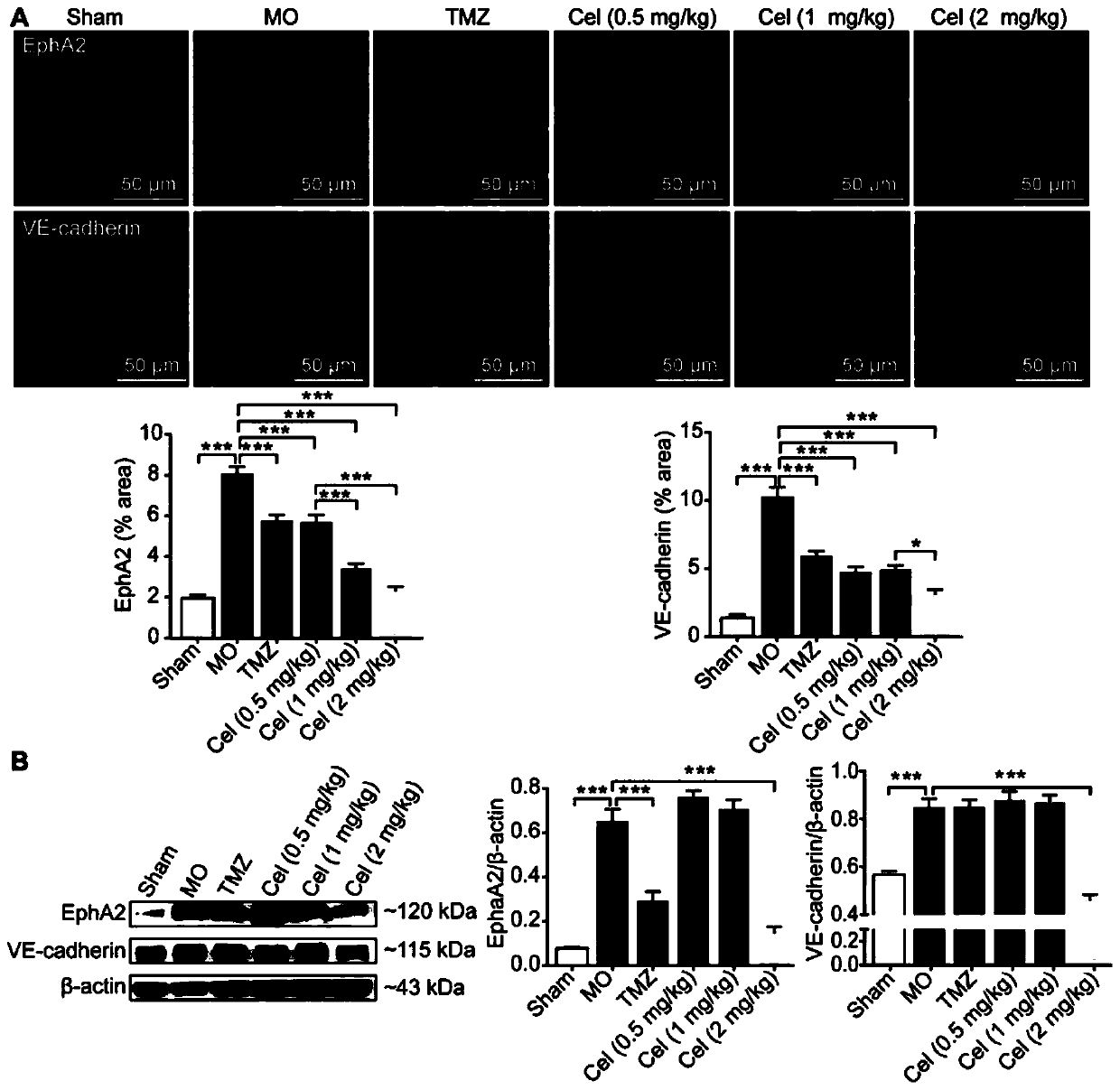
Application of celastrol in inhibiting tumor vasculogenic mimicry
A technology of tumor angiogenesis and tripterine, applied in antineoplastic drugs, organic active ingredients, medical preparations containing active ingredients, etc., can solve the problems that targeted therapy cannot effectively improve patient survival and the mechanism of action is unknown
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 experimental material and method
[0029] 1. Experimental materials
[0030] 1) Drug preparation
[0031] Dissolve 8.05 mg of tripterycin in 402.5 μl of DMSO, filter and sterilize with a 0.22 μm microporous membrane to make a mother solution, and dilute with PBS to make a high dose of tripterycin (Celastrol high dose, Cel-H 2mg / kg ) group, medium dose (Celastrol medium dose, Cel-M 1mg / kg) group and low dose (Celastrol low dose, Cel-L 0.5mg / kg) group, the DMSO content was 1%. Temozolomide (TMZ) 35 mg was dissolved in 175 μl of DMSO, and then configured into 17.5 ml of TMZ solution (20 mg / kg).
[0032] LY294002: LY294002 is a highly selective inhibitor of PI3K in vivo. When used at a concentration of 50 μM, it can specifically attenuate PI3K activity, but does not inhibit other lipid and protein kinases. In this experiment, 1.5 mg LY294002 was added to 98 μl DMSO to prepare a 50 mM stock solution. Dilute to 50μM with complete medium when used.
[0033] ...
Embodiment 3
[0061] Example 3 Study on the Effect and Mechanism of Tripteryglide in Inhibiting VM Formation in U87 and U251 Glioma Cells
[0062] 3.1 CCK8 method was used to analyze the effect of tripterine on the viability of U87 and U251 cells. see results Figure 6 As shown in A, it can be seen that 0.5, 1 μM can inhibit the proliferation of U87 and U251 cells without affecting the activity of normal astrocytes.
[0063]3.2 Scratch test, Transwell chamber migration and invasion test to observe the effect of optimal dose of tripterine on the migration and invasion of U87 and U251 cells. see results Figure 6 As shown in B-D, tripterine 1 μM can significantly inhibit the migration and invasion of U87 and U251 cells.
[0064] 3.3 Observe the formation of VM in U87 and U251 cells and the influence of VE-cadherin expression under light microscope;
[0065] 1) Observe the formation of VM in U87 and U251 cells
[0066] Experimental preparation: Take out the Matrigel Matrigel stored at -20...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com