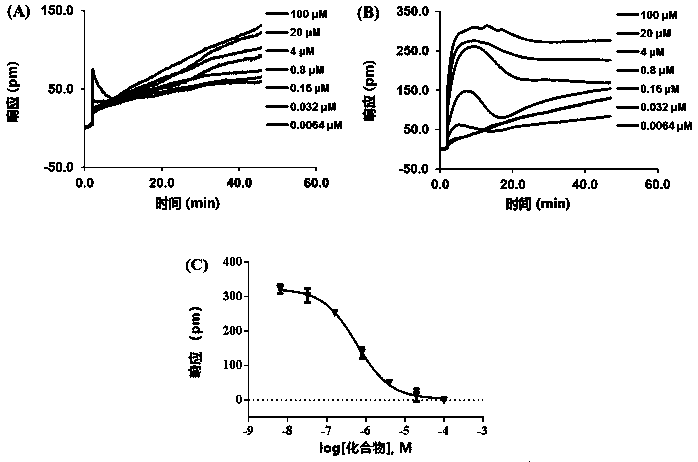
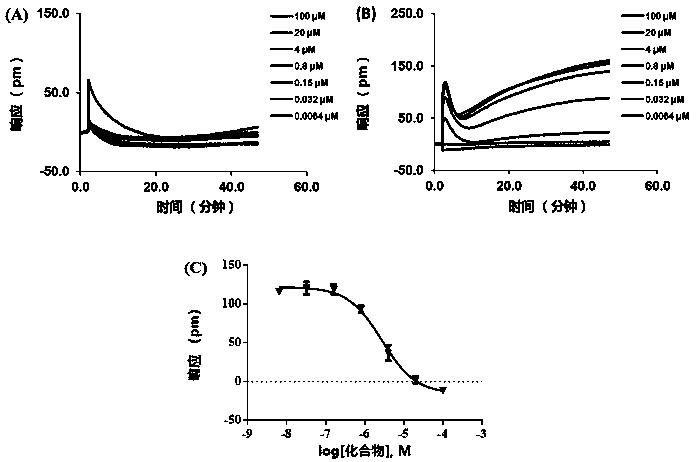
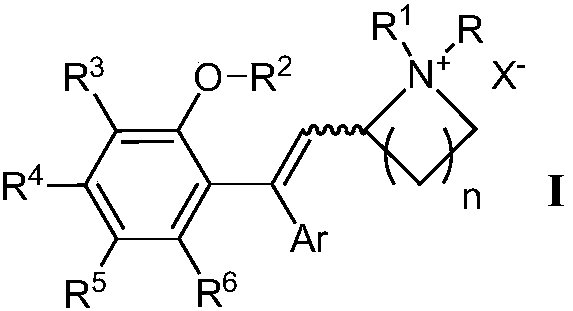
2-(2,2-diarylvinyl)-quaternary ammonium salt type cyclic amine derivative and preparation method thereof
A technology of diarylethene and quaternary ammonium salt, which is applied in the directions of active ingredients of heterocyclic compounds, drug combinations, pharmaceutical formulations, etc. Problems such as few cyclic amine derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0140] Synthesis of 2-[(E)-2-(2-hydroxy-phenyl)-2-phenyl-vinyl-]-N,N-dimethylpiperidinium bromide (I-1)
[0141] According to the steps described in the synthetic method Scheme 1, 2-[(E)-2-(2-hydroxyl-phenyl)-2-phenyl-vinyl-]-N-methylpiperidine is reacted with methyl bromide to prepare The target product I-1 was obtained as a white solid with a yield of 98%. MS(m / z): 308.2[M+H] + . 1 H NMR (400MHz, CD 3 OD), δ: 7.46-7.43(m, 2H), 7.41-7.39(m, 1H), 7.22-7.20(m, 2H), 7.15-7.11(m, 1H), 6.89(d, J=7.6Hz, 1H),6.83(d,J=6.8Hz,1H),6.77-6.73(m,1H),6.07(d,J=9.6Hz,1H),3,64-3.62(m,1H),3.50-3.46 (m,1H),2.97-2.94(m,1H),2.87(s,3H),2,80(s,3H),2.21-2.18(m,1H),1.93-1.90(m,2H),1.84 -1.77(m,2H),1.58-1.55(m,1H).
Embodiment 2
[0143] 2-[2,2-bis(2-hydroxy-phenyl)-vinyl-]-N,N-dimethylpiperidinium bromide (I-2 and its chiral monomer I-2-1, I -2-2) Synthesis
[0144] According to the steps described in the synthetic method Scheme 1, the target product I-2 can be obtained by reacting 2-[2,2-bis(2-hydroxy-phenyl)-vinyl]-N-methylpiperidine with methyl bromide, Yield 95%. MS(m / z): 324.2[M+H] + . 1 H NMR (400MHz, CD 3 OD), δ: 7.33(d, J=4Hz, 1H), 7.18-7.14(m, 1H), 7.12-7.09(m, 1H), 6.97-6.92(m, 2H), 6.85-6.80(m, 2H ),6.78-6.73(m,1H),4.52(q,J=10.4Hz,1H),2.99(d,J=13.2Hz,1H),2.74(b,1H),2.54(b,1H),2.43 (s, 3H), 2.39 (s, 3H), 2.17-2.08 (m, 2H), 1.76-1.60 (m, 2H), 1.50-1.42 (m, 2H), 1.41-1.31 (m, 2H).
[0145] A pair of enantiomeric monomers of 2-[2,2-bis(2-hydroxy-phenyl)-vinyl]-N-methylpiperidine are respectively reacted with methyl bromide to obtain the target product Sexual monomer compound I-2-1 or I-2-2.
[0146] Through HPLC chiral analysis, the retention times of I-2-1 and I-2-2 are: 14.4min and 20....
Embodiment 3
[0148] 2-[(E)-2-(2-Hydroxy-5-methyl-phenyl)-2-phenyl-vinyl-]-N,N-dimethylpiperidinium bromide / iodide (I- 3-Br, I-3-I, and their chiral monomers) synthesis:
[0149] According to the steps described in the synthetic method Scheme 1, 2-[(E)-2-(2-hydroxyl-5-methyl-phenyl)-2-phenyl-vinyl-]-N-methylpiperidine and The white solid target product I-3-Br or I-3-I can be obtained by reacting methyl bromide or methyl iodide with a yield of 99%. MS(m / z): 322.2[M+H] + . 1 H NMR (400MHz, CD 3 OD), δ: 7.36-7.32 (m, 5H), 7.11-7.09 (m, 1H), 6.87-6.85 (m, 1H), 6.81-6.80 (m, 1H), 6.08 (d, J=9.6Hz, 1H),3.80-3.72(m,1H),3.53-3.46(m,1H),3.33-3.22(m,1H),3.14(s,3H),2.94(s,3H),2.27(s,3H) ,2.15-2.05(m,2H),2.0-1.86(m,2H),1.85-1.75(m,1H),1.55-1.43(m,1H).
[0150] A pair of enantiomeric monomers of 2-[(E)-2-(2-hydroxy-5-methyl-phenyl)-2-phenyl-vinyl-]-N-methylpiperidine Respectively react with methyl bromide to obtain the target chiral monomer compound I-3-Br-1 or I-3-Br-2.
[0151] Through HPLC ch...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com