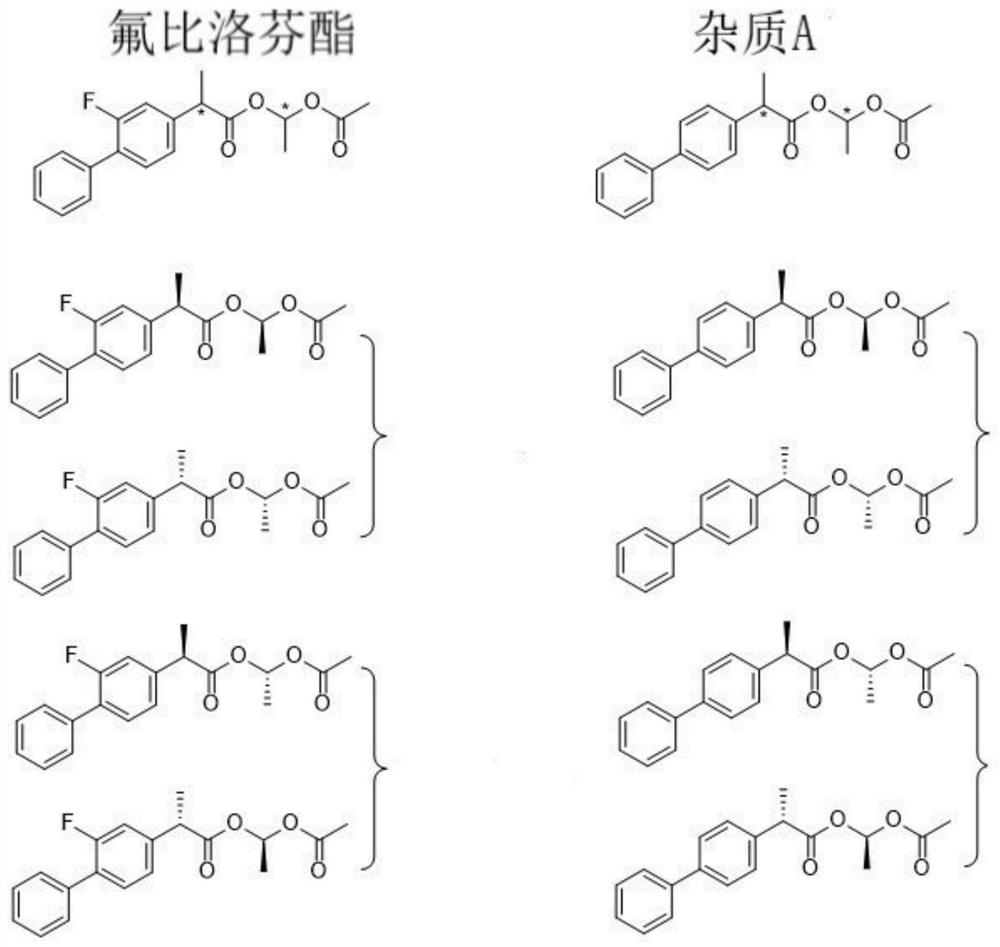
Flurbiprofen axetil enantiomer and liquid chromatography separation detection method of impurity a
A technology for flurbiprofen axetil and enantiomer, which is applied in the field of liquid chromatography separation and detection of flurbiprofen axetil enantiomer and impurity A, can solve the problem that separation results are not described in detail and chromatographic methods are complex and other problems, to achieve the effects of good reproducibility and stability, simple and easy chromatographic separation, and stable separation results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
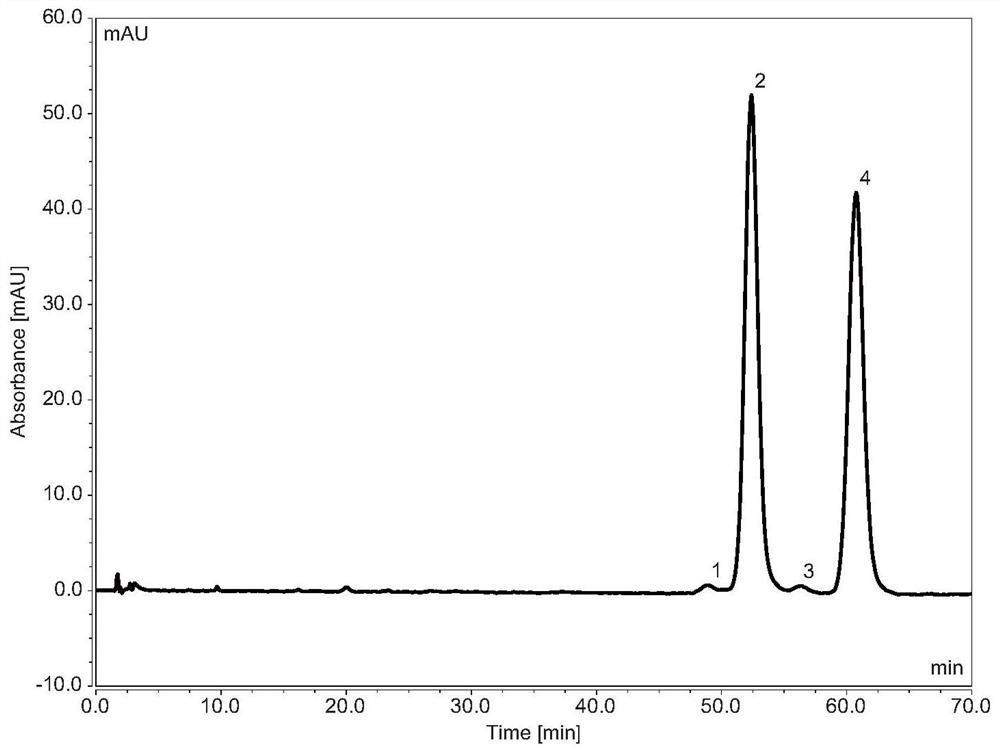
[0045] Embodiment 1 uses the inventive method to separate and detect flurbiprofen axetil and its impurity A
[0046]1) Flurbiprofen axetil stock solution is diluted, as need testing solution; Get impurity A material dilution, as impurity A contrast solution; Get described need test solution and impurity A contrast solution mixing, as system suitability solution ;
[0047] 2) Two chromatographic columns using octadecylsilane bonded silica gel as filler and pentafluorophenylsilane bonded silica gel as filler;
[0048] 3) Using methanol and phosphoric acid aqueous solution as mobile phase, elution mode: isocratic elution.
[0049] Chromatographic conditions:
[0050] Chromatographic column: ChromCore C18 5μm (4.6×100mm) and ChromCore PFP 5μm (4.6×50mm) used in series
[0051] Mobile phase: 60 / 40 methanol / 0.1% phosphoric acid in water v / v
[0052] Column temperature: 30°C
[0053] Flow rate: 1mL / min
[0054] Injection volume: 5μL
[0055] Detection wavelength: 254nm
[005...
Embodiment 2
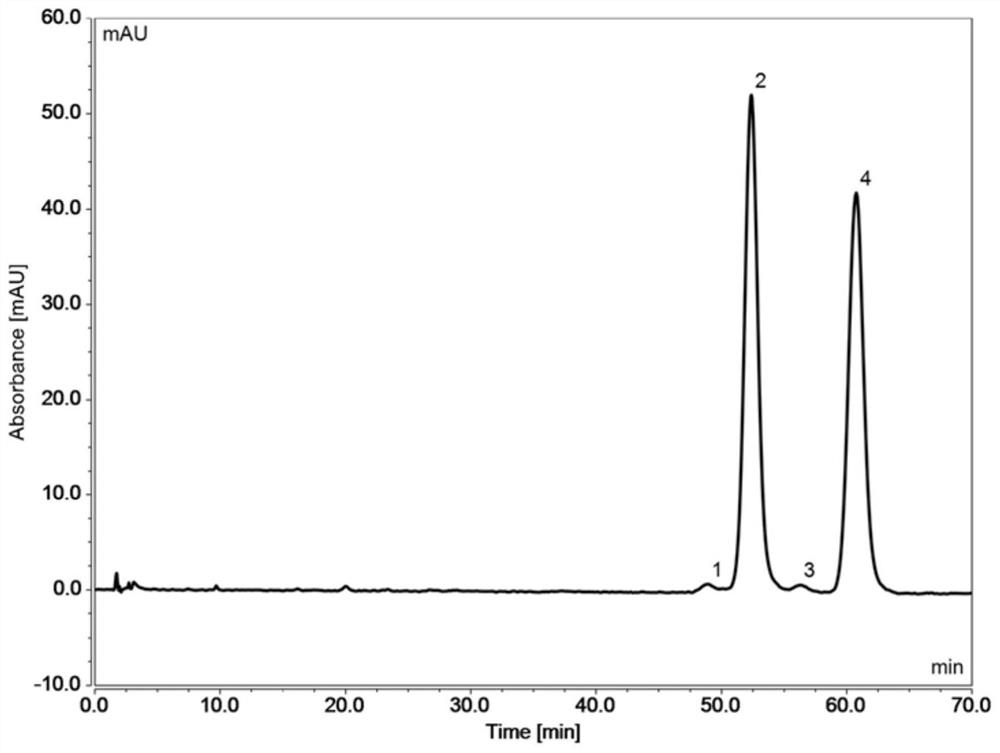
[0061] Embodiment 2 adopts the same chromatographic column and mobile phase of Embodiment 1, only the column temperature is different, and the enantiomers of flurbiprofen axetil and the corresponding isomers of impurity A are separated and detected at 20°C and 40°C respectively .
[0062] Chromatographic conditions:
[0063] Chromatographic column: ChromCore C18 5μm (4.6×100mm) and ChromCore PFP 5μm (4.6×50mm) used in series
[0064] Mobile phase: 60 / 40 methanol / 0.1% phosphoric acid in water v / v
[0065] Column temperature: 20°C / 40°C
[0066] Flow rate: 1mL / min
[0067] Injection volume: 5μL
[0068] Detection wavelength: 254nm
[0069] The preparation of need testing solution: get flurbiprofen axetil stock solution 10 μ L, add methanol 20mL dilution, as need testing solution; Get impurity A material solution 10 μ L, add methanol 20mL dilution, as impurity A contrast solution; Take need testing solution 1mL and impurity A control solution 10μL, mix well, as the system su...
Embodiment 3
[0072] Embodiment 3 adopts the same chromatographic column of embodiment 1, column temperature, mobile phase, only change the ratio of mobile phase methanol / phosphoric acid aqueous solution, separate and detect the enantiomer of flurbiprofen axetil and the corresponding isomer of impurity A
[0073] Chromatographic conditions:
[0074] Chromatographic column: ChromCore C18 5μm (4.6×100mm) and ChromCore PFP 5μm (4.6×50mm) used in series
[0075] Mobile phase: 65 / 35 methanol / 0.1% phosphoric acid in water v / v
[0076] Column temperature: 30°C
[0077] Flow rate: 1mL / min
[0078] Injection volume: 5μL
[0079] Detection wavelength: 254nm
[0080] The preparation of need testing solution: get flurbiprofen axetil stock solution 10 μ L, add methanol 20mL dilution, as need testing solution; Get impurity A material solution 10 μ L, add methanol 20mL dilution, as impurity A contrast solution; Take need testing solution 1mL and impurity A control solution 10μL, mix well, as the syst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com