Polypeptides binding adamts5, mmp13 and aggrecan
A technology of MMP13 and polyprotein, applied in the direction of fusion polypeptide, immunoglobulin, peptide/protein components, etc., can solve unwanted problems, achieve the effect of reducing burden and increasing effective dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
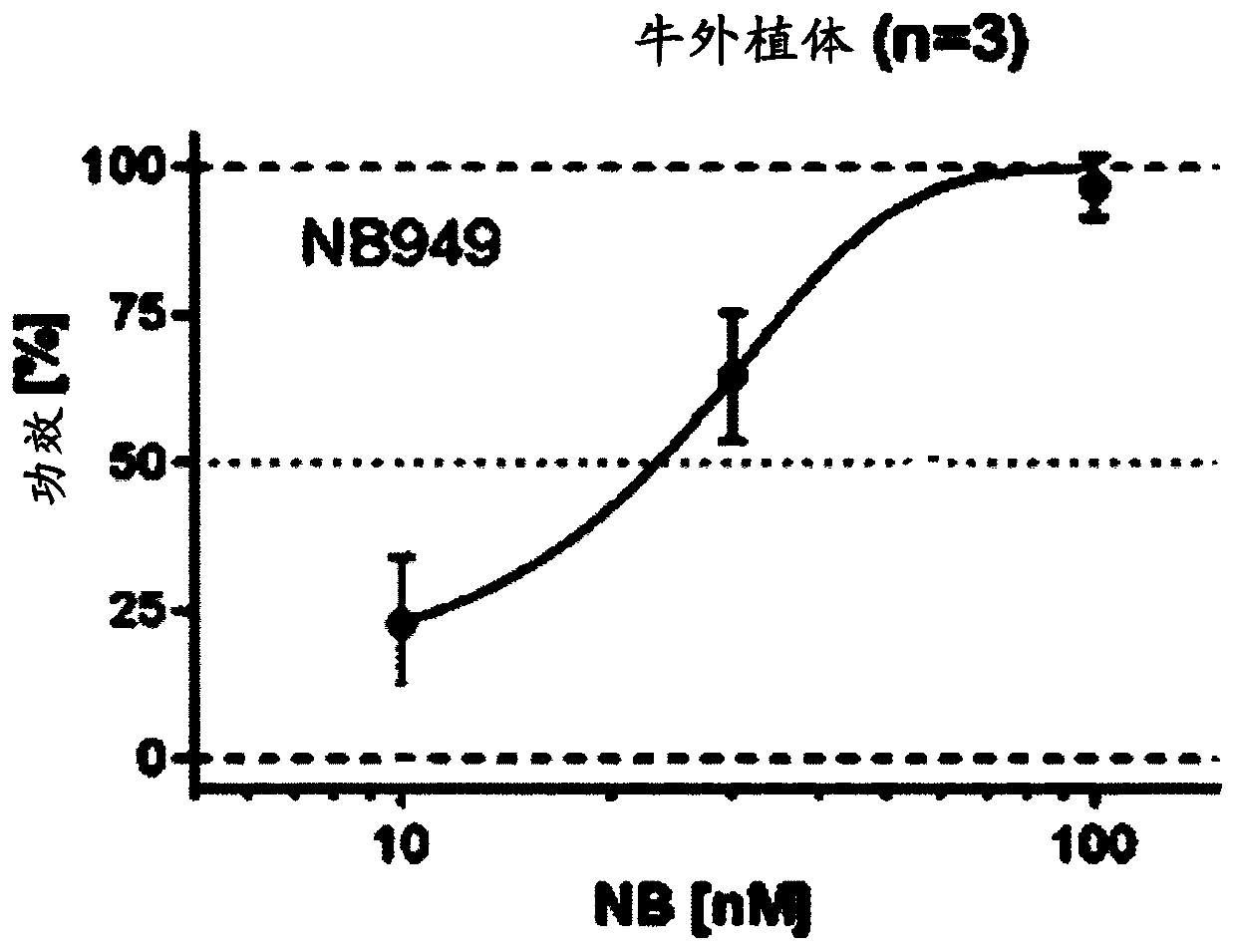
[0397] Example 1 MMP13 ISVD
[0398] 1.1 Anti-MMP13 ISVD 62C02
[0399] After screening more than 10E7 clones, the MMP13-specific ISVD 62C02 was identified in a fluorescent peptide assay, a collagen lysis assay, and a fluorescent collagen assay.
[0400] Briefly, the human, cynomolgus, rat, dog, and bovine MMP13 fluorogenic peptide assays, and the human MMP1 and MMP14 fluorogenic peptide assays were set up as follows: activated MMPs were mixed with the fluorogenic peptide substrate Mca-PLGL-Dpa-AR- NH2 (R&D Systems #ES001) and 1 / 5 dilution of periplasmic extract or serial dilution of purified Nanobody / positive control (total volume = 20 μl in assay buffer 50 mM Tris pH 7.5, 100 mM NaCl, 10 mM CaCl , 0.01% Tween20) were incubated at 37°C for 2h. The linear increase in fluorescence (v0 - 15 to 45 min incubation) was used as a measure of enzyme activity and using Equation 100 - 100 (v0 in the presence of test Nanobodies / v0 in the presence of negative control Nanobodies (Cablys)...
Embodiment 2
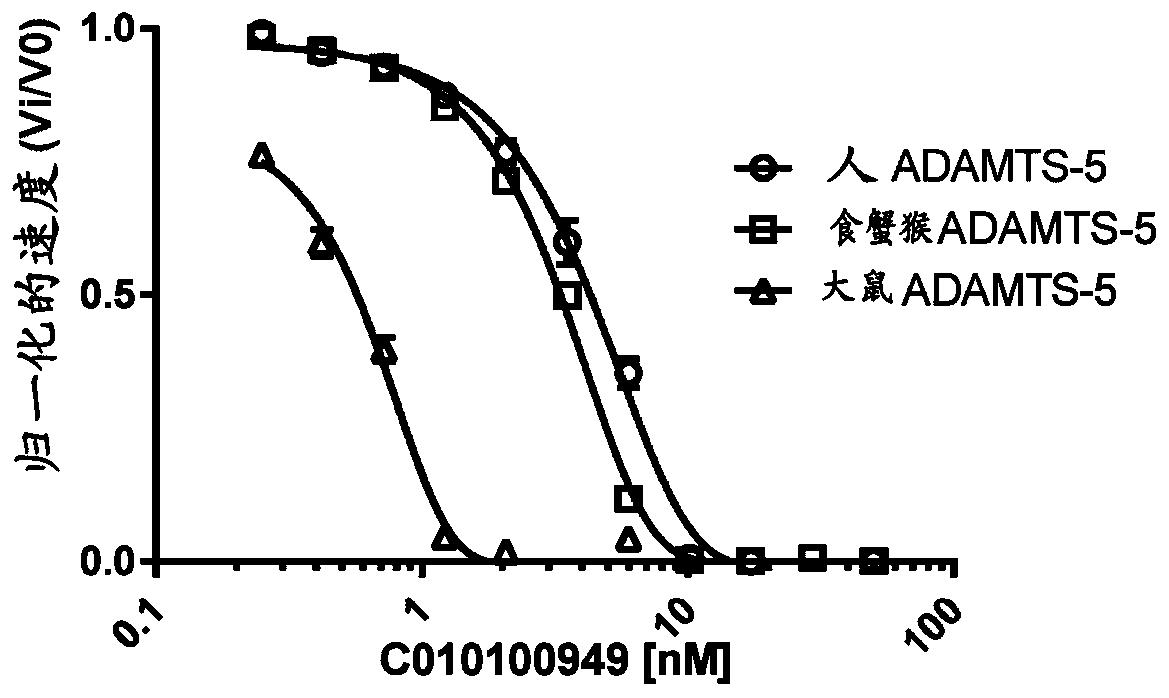
[0410] Example 2 ADAMTS5 ISVD
[0411] 2.1 Anti-ADAMTS5 ISVD 02F03
[0412] Also in this case, more than 10E7 clones were screened to identify ISVD02F03 that specifically binds ADAMTS5. ISVD 02F03 was also characterized by inhibition of ADAMTS5-mediated aggrecan cleavage by FRET and AlphaLISA based assays.
[0413] Briefly, periplasmic extracts of ISVD 02F03 were tested for binding to recombinant human ADAMTS5 by binding ELISA. Next, it was demonstrated that ISVD 02F03 was able to prevent ADAMTS5-mediated aggrecan cleavage in a FRET-based enzymatic assay for human ADAMTS5. Following the FRET-based assay, an AlphaLISA (Perkin Elmer, Waltham, MA, US)-based human ADAMTS5 assay was performed with a biotinylated 43-mer aggrecan oligopeptide as substrate. Following cleavage of this substrate by ADAMTS5, the biotinylated ARGSV neo-epitope product is released and can be used with streptavidin-AlphaScreen donor beads and α-neo-captured on anti-mouse IgG-coated AlphaLISA acceptor bea...
Embodiment 3
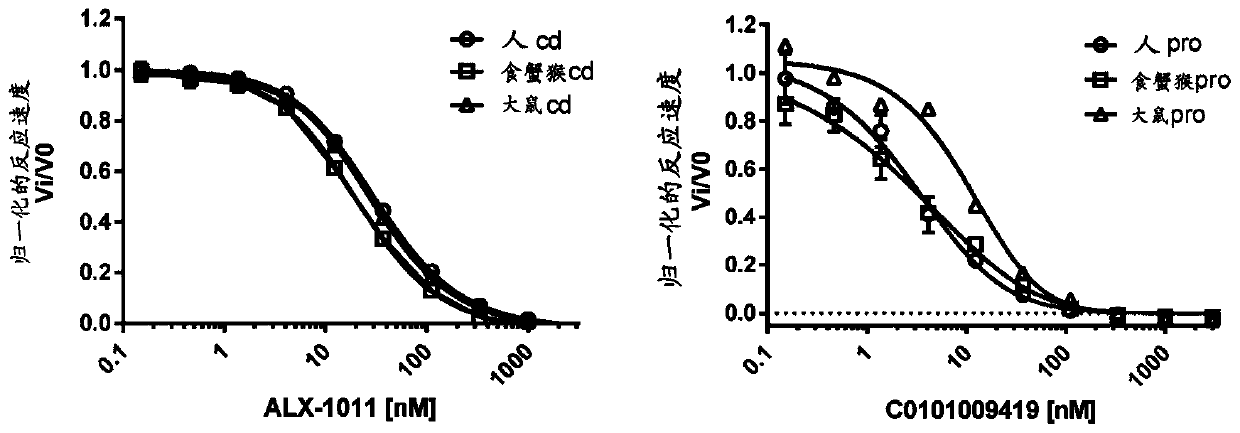
[0435] Example 3: Aggrecan ISVD3.1 Anti-Aggrecan ISVD 114F08
[0436] After an extensive screening campaign, the aggrecan-specific ISVD 114F08 was isolated and characterized. Immunization of llamas with recombinant human aggrecan (G1-IGD-G2 domain, R&D Systems #1220-PG) produced specificity and high serum titers. However, only a small fraction of isolated nanobodies meet the two requirements of binding to the G1 domain of aggrecan and showing broad species cross-reactivity. After painstaking efforts, multiple family members were identified that displayed essentially similar features (see Table 3.1A to Table 3.1C for sequence changes in the CDRs). Ultimately, ISVD 114F08 ("C0101PMP114F08") was selected for further characterization.
[0437] Table 3.1.1 provides an overview of the domain mapping and species cross-reactivity data.
[0438]
[0439] After primary screening, initial assessment of binding by ELISA, determination of off-rates and species cross-reactivity, ISVD ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| affinity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com