2-cyano-3-thiophene substituted valeramide derivatives and their applications
A compound, methoxyl technology, applied in the field of pesticides, to achieve the effects of novel structure, good control effect and excellent activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
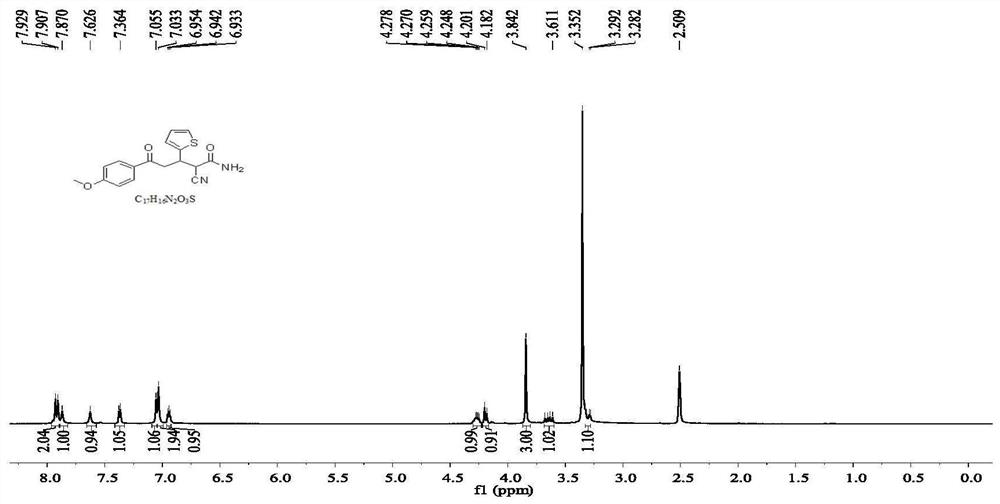
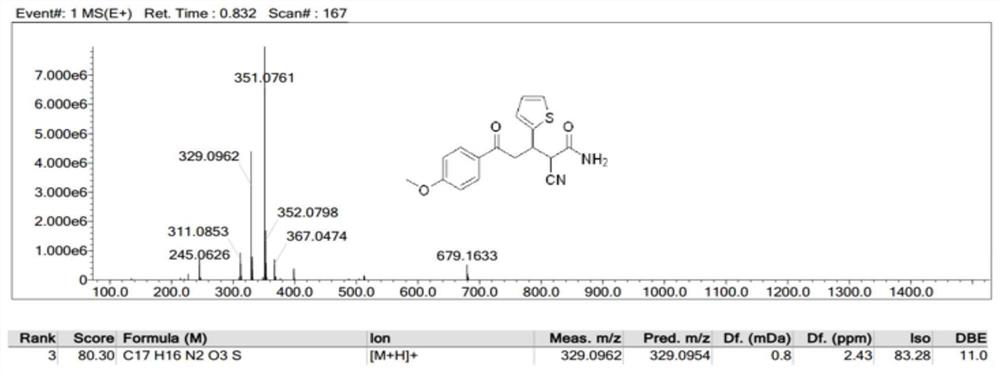
[0059] compound preparation of
[0060] 0.01 mol of 4-methoxyacetophenone was dissolved in 10 mL of absolute ethanol, and 5 mL of 10% NaOH ethanol solution was added thereto. While stirring in an ice bath, slowly drop the mixture of 0.01mol thiophene-2-carbaldehyde and 10mL of absolute ethanol into the above mixed solution with a constant pressure dropping funnel, react at 0-5°C, and use a thin-layer silica gel plate to (TLC) to check the completion of the reaction. After the reaction is completed, add 5 mL of 10% NaOH ethanol solution to the reaction mixture, and slowly drop a mixed solution of 0.02 mol cyanoacetamide and 10 mL of absolute ethanol into the mixture with a constant pressure dropping funnel, and continue to cool at 0-5 ° C. The reaction was carried out, and TLC was used to check whether the reaction was complete. After the reaction was completed, a large amount of ice water was added to the solution, and the pH was adjusted to neutral with 10% hydrochloric a...
Embodiment 2
[0062] compound preparation of
[0063] 0.01 mol of 4-bromoacetophenone was dissolved in 10 mL of absolute ethanol, and 5 mL of 10% NaOH ethanol solution was added thereto. While stirring in an ice bath, slowly drop the mixture of 0.01mol thiophene-2-carbaldehyde and 10mL of absolute ethanol into the above mixed solution with a constant pressure dropping funnel, react at 0-5°C, and use a thin-layer silica gel plate to (TLC) to check the completion of the reaction. After the reaction is completed, add 5 mL of 10% NaOH ethanol solution to the reaction mixture, and slowly drop a mixed solution of 0.02 mol cyanoacetamide and 10 mL of absolute ethanol into the mixture with a constant pressure dropping funnel, and continue to cool at 0-5 ° C. The reaction was carried out, and TLC was used to check whether the reaction was complete. After the reaction was completed, a large amount of ice water was added to the solution, and the pH was adjusted to neutral with 10% hydrochloric aci...
Embodiment 3
[0066] compound preparation of
[0067] 0.01 mol of 4-methoxyacetophenone was dissolved in 10 mL of absolute ethanol, and 5 mL of 10% NaOH ethanol solution was added thereto. Under stirring in an ice bath, slowly drop a mixture of 0.01mol 5-bromothiophene-2-carbaldehyde and 10mL of absolute ethanol into the above mixed solution with a constant pressure dropping funnel, react at 0-5°C, and use a thin A silica gel plate (TLC) was used to check whether the reaction was complete. After the reaction is complete, add 5 mL of 10% NaOH ethanol solution to the reaction mixture, and slowly drip a mixed solution of 0.02 mol cyanoacetamide and 10 mL of absolute ethanol into the mixture with a constant pressure dropping funnel, and continue to The reaction was carried out at ℃, and the completion of the reaction was detected by TLC. After the reaction was completed, a large amount of ice water was added to the solution, and the pH was adjusted to neutral with 10% hydrochloric acid solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com