A method for constructing the ULC characteristic map of the soil fritillaria medicinal material and its component content determination method
A technology of characteristic map and construction method, which is applied in the field of construction of UPLC characteristic map of Tubeimu medicinal material, can solve the problems of difficulty in fully reflecting the internal quality of the medicinal material, limited separation ability, lack of specificity, etc., and achieve stable specificity, fast method, and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
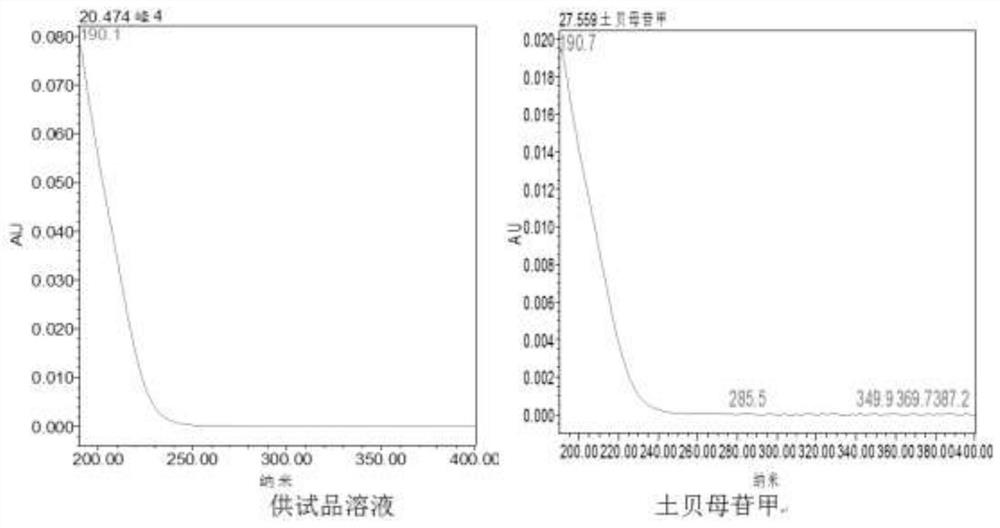
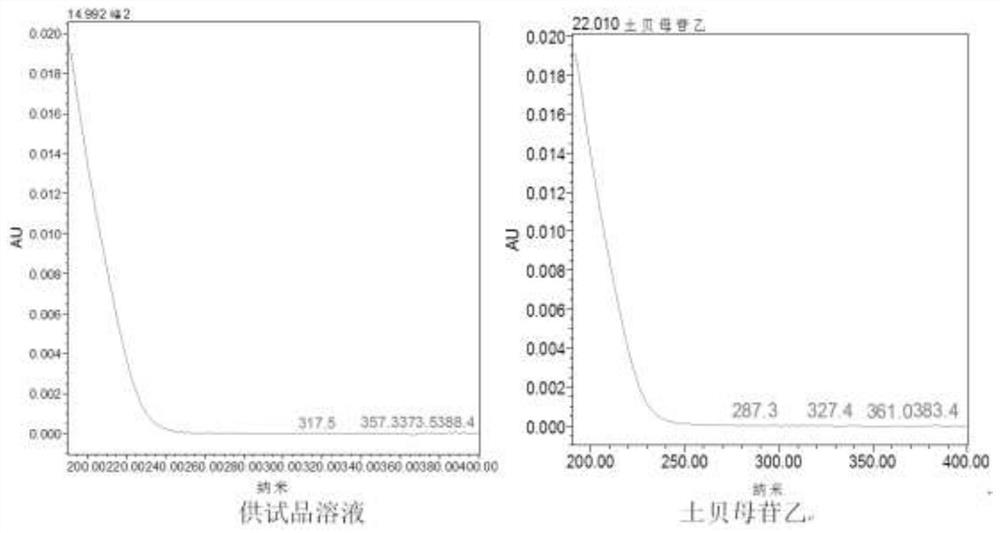
[0042] Construction Method of UPLC Characteristic Map of Soil Fritillaria Medicinal Material
[0043] 1. The chromatographic conditions for ultra-high performance liquid chromatography analysis are as follows: use a WatersCortecs T3 chromatographic column with a specification of 2.1×100mm and 1.6 μm, use acetonitrile as mobile phase A, and use 0.1% phosphoric acid aqueous solution as mobile phase B to carry out gradient washing The column temperature is 30°C; the flow rate is 0.3ml / min; the detection wavelength is 203nm, and the injection volume is 1µl.
[0044] 2. Gradient elution conditions are: 0~4min, the volume fraction of mobile phase A changes from 5% to 11%, and the volume fraction of mobile phase B is 95%~89%; 4~7min, the volume fraction of mobile phase A changes 11%~20%, the volume fraction of mobile phase B changes to 89%~80%; 7~21min, the volume fraction of mobile phase A changes to 20%~28%, and the volume fraction of mobile phase B changes to 80%~ 72%; 21~30min, ...
Embodiment 2
[0066] Method for Determination of Component Content in Soil Fritillaria Medicinal Material
[0067] 1. The chromatographic conditions for ultra-high performance liquid chromatography analysis are as follows: use a WatersCortecs T3 chromatographic column with a specification of 2.1×100mm and 1.6 μm, use acetonitrile as mobile phase A, and use 0.1% phosphoric acid aqueous solution as mobile phase B to carry out gradient washing The column temperature is 30°C; the flow rate is 0.3ml / min; the detection wavelength is 203nm, and the injection volume is 1µl.
[0068] 2. Gradient elution conditions are: 0~4min, the volume fraction of mobile phase A changes from 5% to 11%, and the volume fraction of mobile phase B is 95%~89%; 4~7min, the volume fraction of mobile phase A changes 11%~20%, the volume fraction of mobile phase B changes to 89%~80%; 7~21min, the volume fraction of mobile phase A changes to 20%~28%, and the volume fraction of mobile phase B changes to 80%~ 72%; 21~30min, t...
Embodiment 3
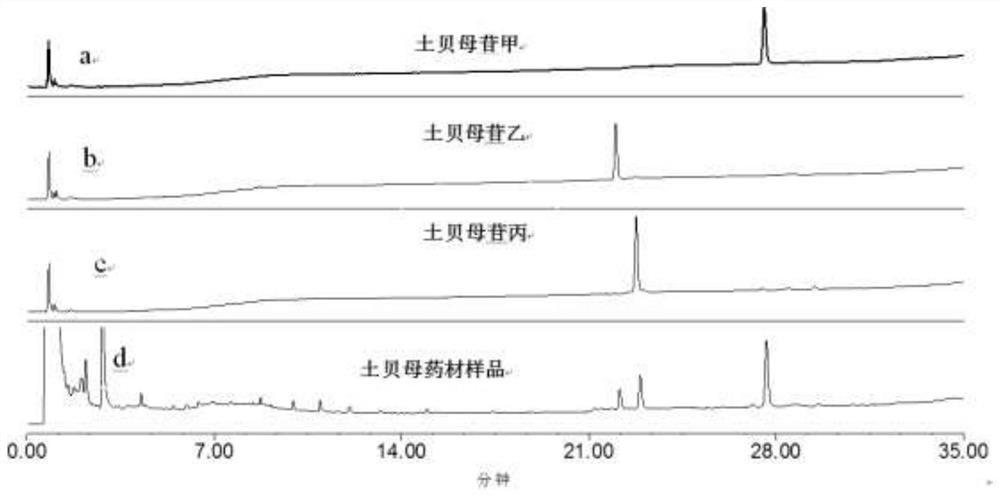
[0103] Using UPLC characteristic chromatogram to identify the medicinal material of Fritillaria japonicus.
[0104] The soil fritillary medicinal material is a medicinal material purchased randomly in the market.
[0105] 1. The chromatographic conditions for ultra-high performance liquid chromatography analysis are as follows: use a WatersCortecs T3 chromatographic column with a specification of 2.1×100mm and 1.6 μm, use acetonitrile as mobile phase A, and use 0.1% phosphoric acid aqueous solution as mobile phase B to carry out gradient washing The column temperature is 30°C; the flow rate is 0.3ml / min; the detection wavelength is 203nm, and the injection volume is 1µl.
[0106] 2. Gradient elution conditions are: 0~4min, the volume fraction of mobile phase A changes from 5% to 11%, and the volume fraction of mobile phase B is 95%~89%; 4~7min, the volume fraction of mobile phase A changes 11%~20%, the volume fraction of mobile phase B changes to 89%~80%; 7~21min, the volume ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com