Application of caffeic acid phenethyl ester in treatment of CAVD
A technology of phenethyl caffeate and calcification disease, applied in the field of prevention and/or treatment of aortic valve calcification and phenethyl caffeate, can solve the problem of poor long-term effect, high perioperative mortality, and aggravated patients Family financial burden and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
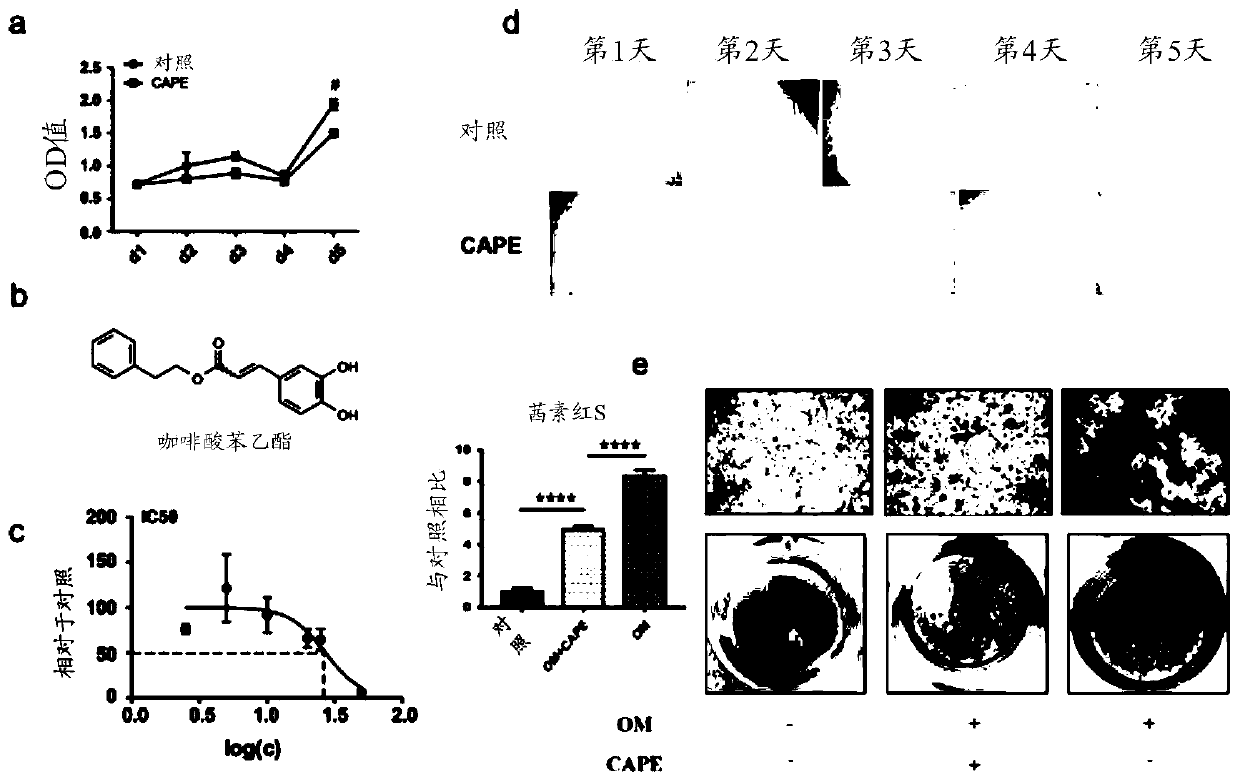
[0022] Example 1. Construction of a human valve mesenchymal cell calcification model induced by a commercial osteogenic induction medium
[0023] Human valve mesenchymal cells with a culture density close to 80% were starved overnight with 2% FBS+DMEM high-sugar culture solution, and then starved with a newly configured osteogenic induction medium (Cyagen, product number: HUXMA-90021). The calcification of the valvular interstitial cells was induced, and alizarin red staining was performed after 21 days of induction to investigate the effects of different treatments on the calcification of the valvular interstitial cells.
Embodiment 2
[0024] Embodiment 2, caffeic acid phenethyl ester inhibits calcification of human valve interstitial cells
[0025] The human valve interstitial cell calcification model constructed as in Example 1 above was adopted, and the human valve interstitial cells were divided into 3 groups, respectively control group (untreated, normal medium), OM group (osteogenic medium induction) and OM+CAPE group (caffeic acid phenethyl ester was added after osteogenic medium induction). Different concentrations of phenethyl caffeate (phenethyl caffeate was purchased from Selleck, catalog number: S7414) were used to treat human valve interstitial cells, and IC50 test, CCK8 determination and Alizarin red staining analysis were performed.
[0026] IC50 results as figure 1 As shown in c, CAPE showed clear signs of toxicity when the concentration exceeded 10 μM, therefore, 10 μM CAPE was used for further experiments. cck8 cell viability detection, the concentration of 10 μM caffeic acid phenethyl es...
Embodiment 3
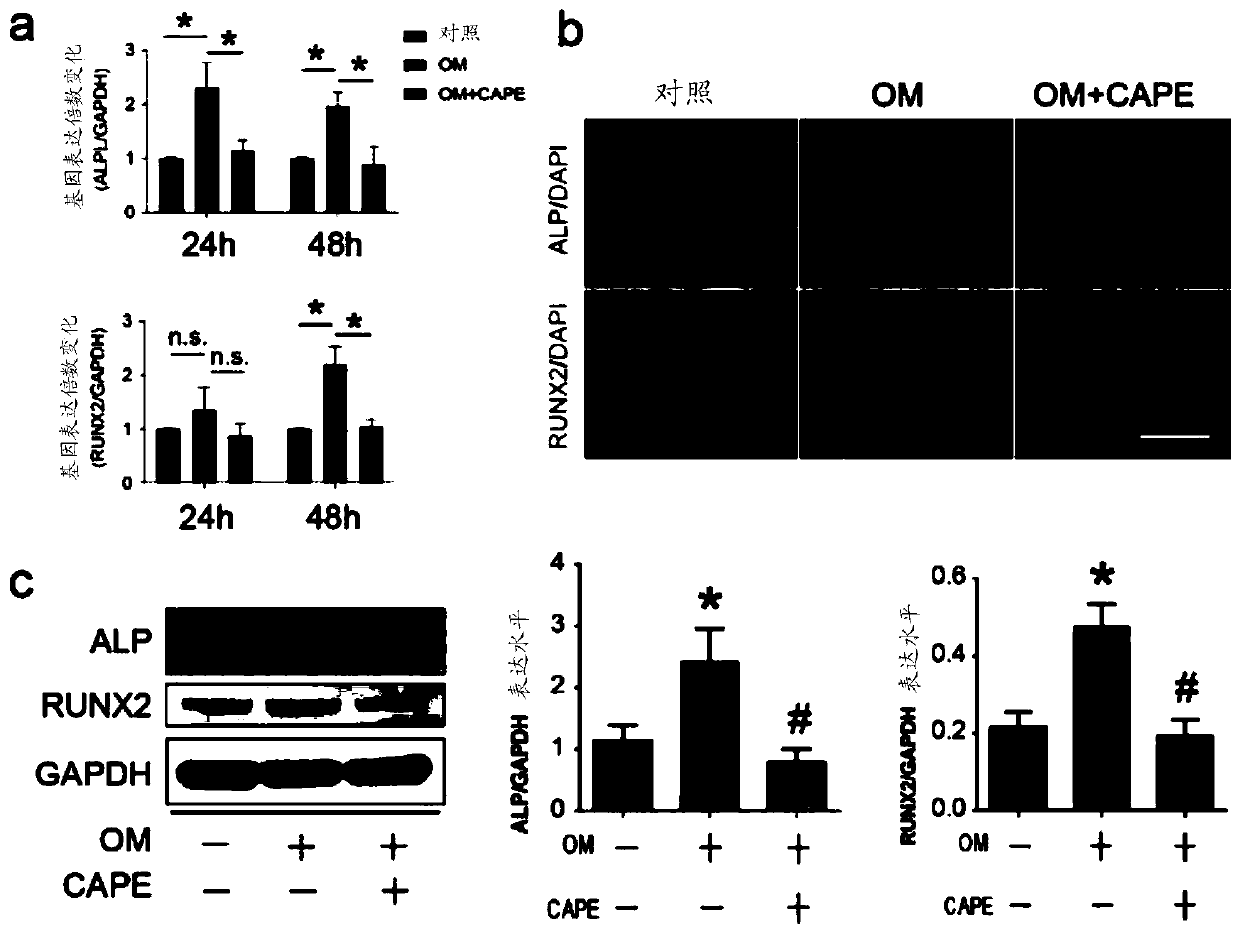
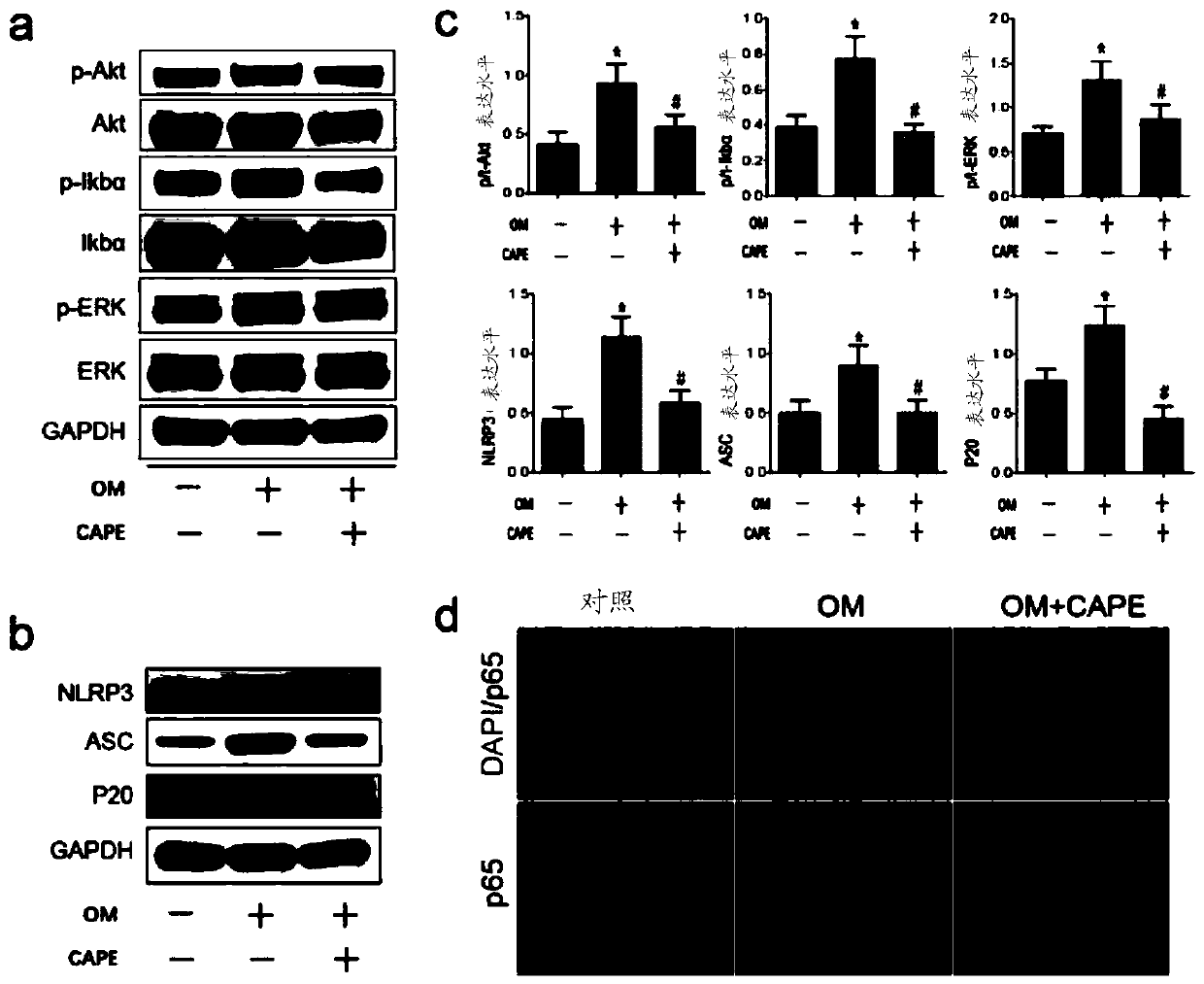
[0027] Example 3, the gene protein expression of phenethyl caffeate inhibiting the calcification of human valve interstitial cells
[0028] The human valve interstitial cell calcification model constructed as in Example 1 above was adopted, and the human valve interstitial cells were divided into 3 groups, respectively control group (untreated, normal medium), OM group (osteogenic medium induction) and OM+CAPE group (caffeic acid phenethyl ester was added after osteogenic medium induction). Human valve mesenchymal cells were treated with 10 μM caffeic acid phenethyl ester at a final concentration (final concentration in culture medium), and osteogenesis-specific genes RUNX2 and ALP were detected at 24h and 48h.
[0029] Quantitative qRT-PCR results showed that the expression of ALP and RUNX2 (osteogenic differentiation marker gene) was significantly upregulated by osteogenic medium compared with the control group (*p figure 2 a), showing a statistically significant difference ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com