Anti-PD-L1 monoclonal antibody, and application thereof in preparation of anticancer drugs
A monoclonal antibody, PD-L1 technology, applied in the biological field, can solve the problem of no PD-L1 antibody drug, and achieve the effect of good affinity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Example 1 Preparation of anti-PD-L1 monoclonal antibody of the present invention
[0031] Technical route of the present invention is:
[0032] After immunizing mice with PD-L1 protein, isolate the mouse spleen, extract total RNA, reverse transcribe into cDNA, and amplify antibody light and heavy chain genes by PCR, and then construct a single-chain antibody phage library , Obtain the target antibody through screening.
[0033] 1. Experimental materials
[0034] 1. Strain E.coli TG1; E.coli DH5α, used for the expression of Fab antibody. Laboratory preservation.
[0035] 2. Plasmid vector pComb3XTT. Laboratory preservation.
[0036] 3. Reagents Trizol total RNA extraction kit, M-MLV reverse transcriptase, and RNase inhibitors were purchased from Invitrogen.
[0037] Tag DNA polymerase, recombinant ligase and endonuclease were purchased from NEB Company. DNA gel recovery kit was purchased from Shanghai Sangong Company.
[0038] 2. Experimental steps
[0039] 1. I...
Embodiment 2
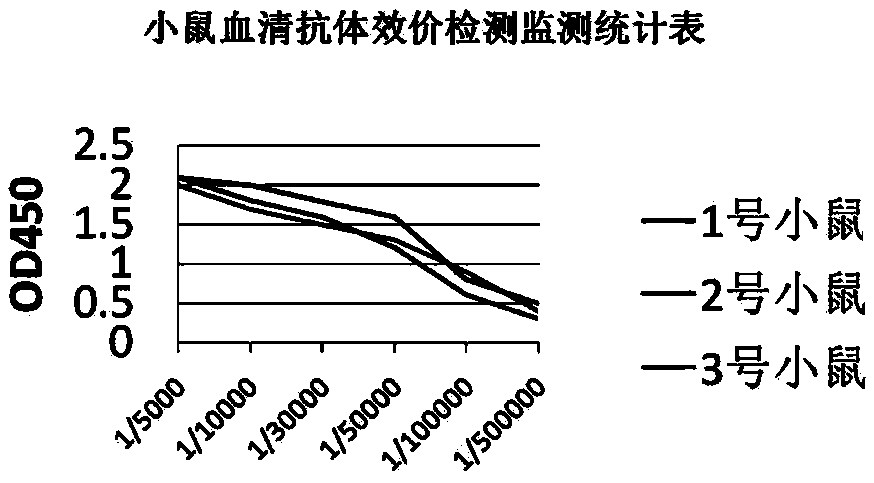
[0074] Example 2 Detection of anti-PD-L1 monoclonal antibody
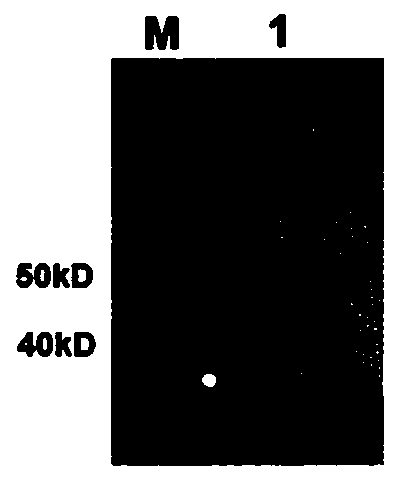
[0075] 1. Antibody Western blot detection
[0076] (1) Preparation of polyacrylamide gel: configure 12% separation gel liquid pure water to solidify after 15 minutes of sealing, then add concentrated gel and insert the corresponding comb, and it can solidify and use after 15 minutes. It is ready to use now to ensure the experimental effect .
[0077] (2) Treat the sample: Dilute an appropriate amount of PD-L1 to 40 μL, add 5x deformation non-reducing buffer at a ratio of 4:1, boil in a metal bath at 100°C for 5 minutes, and centrifuge at 12,000 rpm for 5 minutes.
[0078] (3) Electrophoresis: Add 20 μL of sample to each well, add 5 μL of Rainbow Marker as a control, and perform electrophoresis at a constant current of 50 mA until the indicator reaches the bottom of the gel plate.
[0079] (4) Membrane transfer: Cut off the glue at the size of the target protein according to the appropriate area according to the r...
Embodiment 3
[0095] Example 3 Blocking effect of anti-human PD-L1 monoclonal antibody on PD-1 / PD-L1 interaction
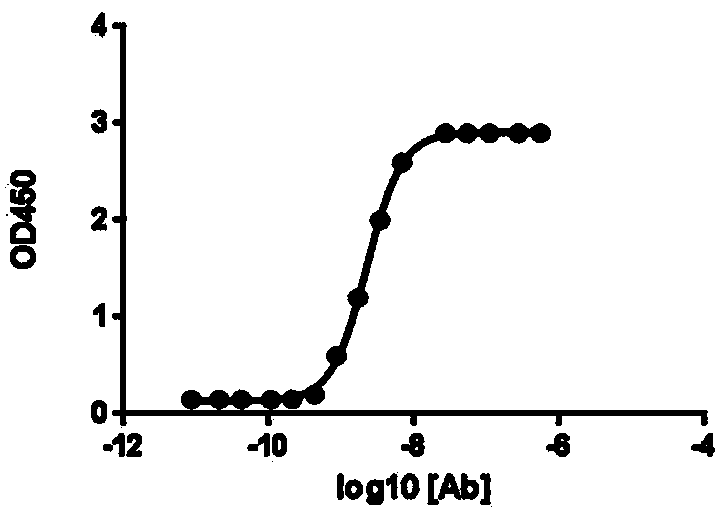
[0096] CHO cells expressing human PD-L1 were suspended in FACS buffer. Add the anti-PD-L1 antibody (0.5 ug / ml) prepared by the present invention to the cell suspension and incubate at 4°C for 30 minutes. Unbound antibody was washed away and FITC-labeled PD-1 recombinant protein (1 ug / ml) was added to the tube and incubated at 4°C for 30 minutes.
[0097] Flow cytometric analysis was performed using a FACScan flow cytometer. According to the measurement of the mean fluorescence intensity (MFI) of the staining, the results showed that the anti-PD-L1 monoclonal antibody blocked the binding of PD-1 to the CHO cells expressing PD-L1, Such as Figure 4 shown. It is proved that the anti-PD-L1 monoclonal antibody prepared by the present invention can significantly inhibit the interaction between PD-1 and PD-L1 on the cell surface.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com