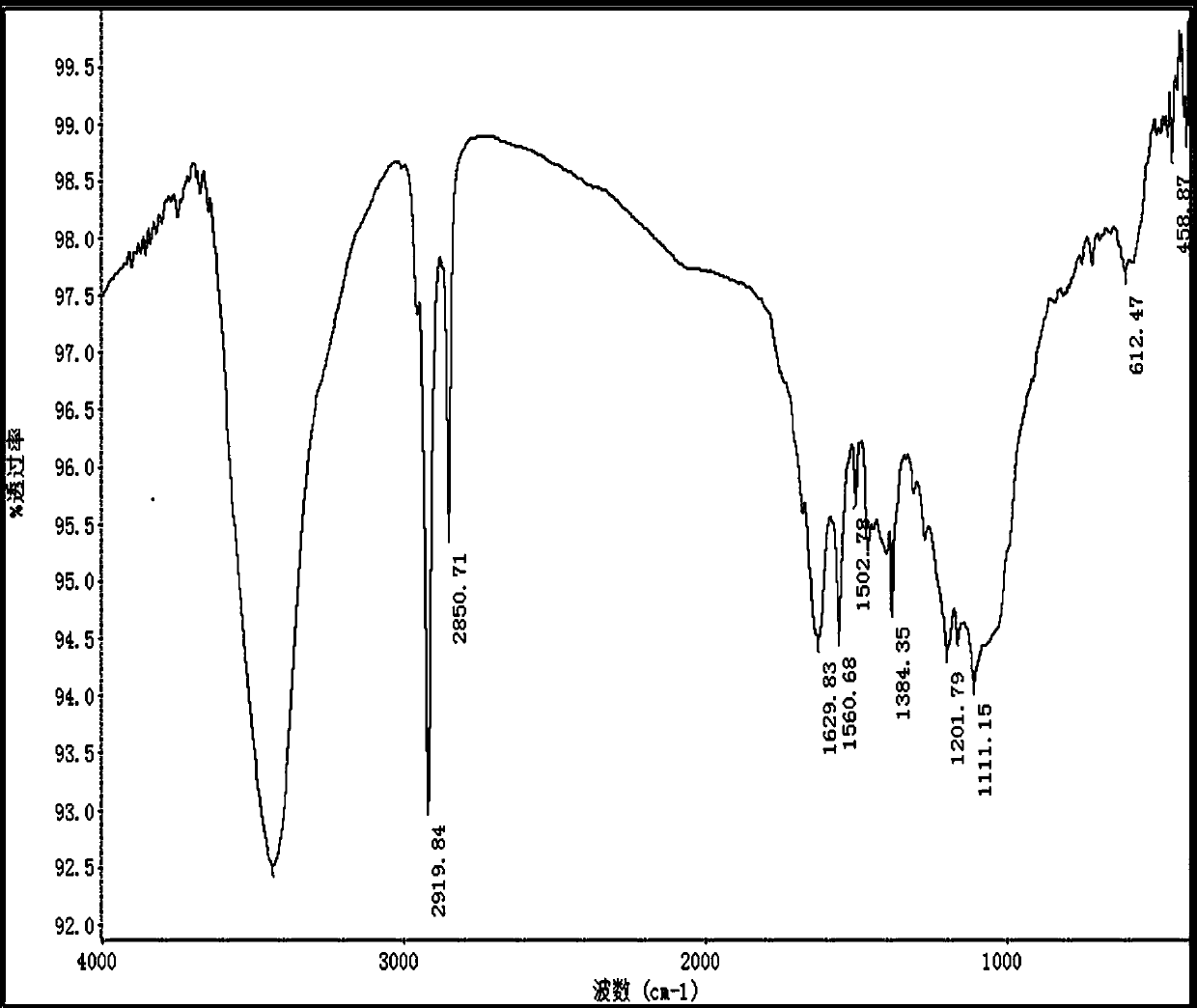
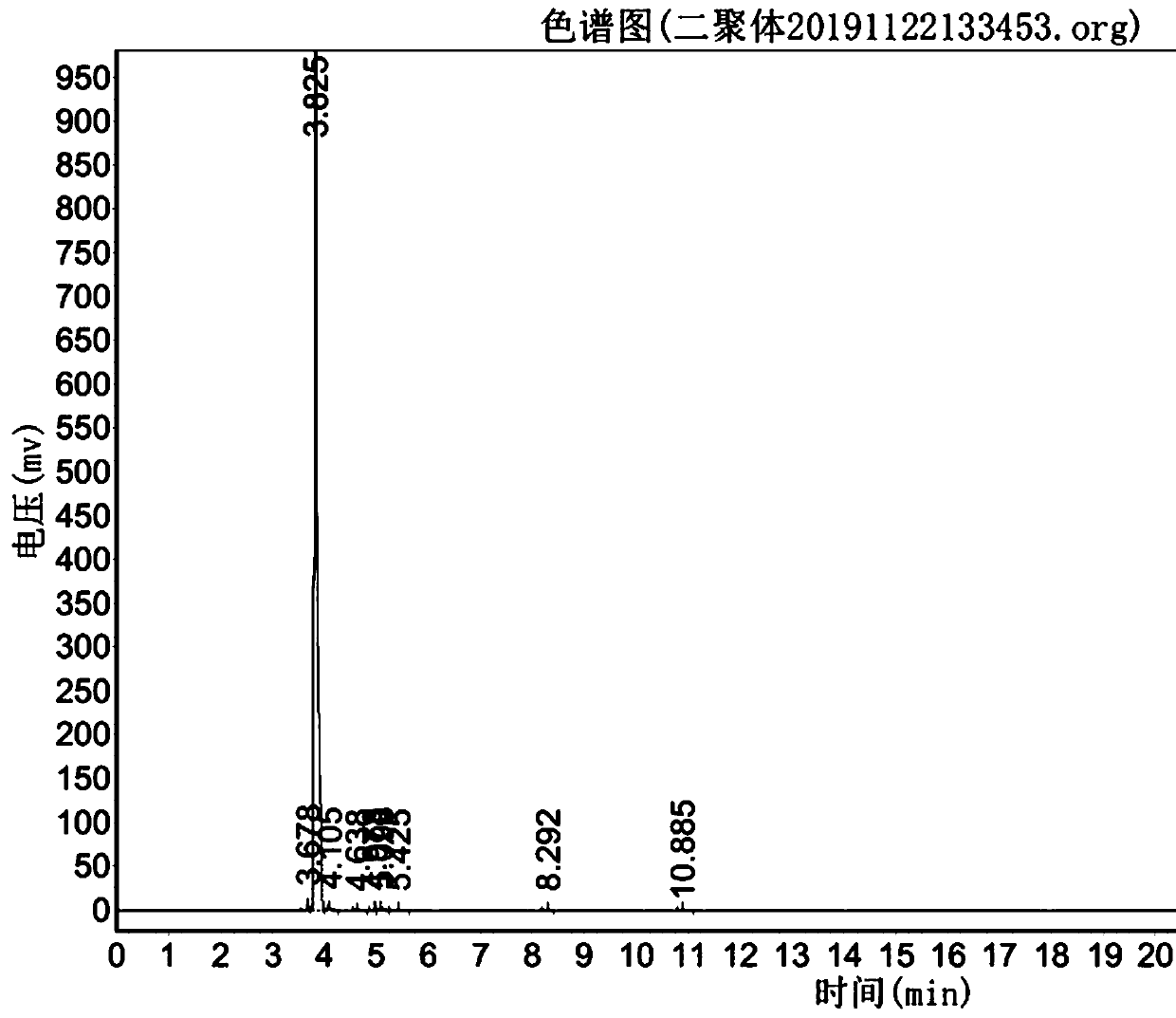
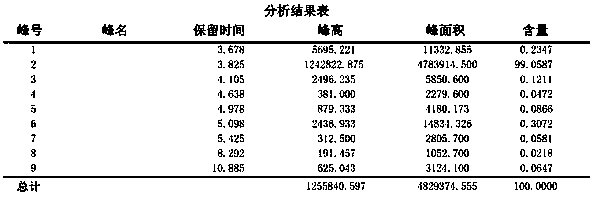
Preparation method of D2 structure hexafluoropropylene dimer with high reaction activity
A technology of hexafluoropropylene dimer and high reactivity, which is applied in the preparation of halogenated hydrocarbons, chemical instruments and methods, organic compounds/hydrides/coordination complex catalysts, etc., can solve problems such as difficult reactions, and achieve low cost The effect of low cost and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] A highly reactive D2 structure hexafluoropropylene dimer is prepared according to the following method:
[0024] Add 0.01Kg of oligomerization catalyst, 0.01Kg of structural isomerization aid and 50Kg of organic solvent into the reaction kettle, stir and mix evenly, use argon to bubble for 5 minutes, then vacuumize, and cycle 3 times with argon, under vacuum condition Charge 15Kg of hexafluoropropylene at the bottom, control the temperature at 30°C, stir and react for 2 hours, after completing the reaction, cool down to 5°C, then discharge the residual hexafluoropropylene gas, separate the liquid to obtain the lower layer liquid, add it to the high-pressure reactor, and add 0.02Kg of metal Fluoride and 0.05Kg structural isomerization additive, heated to 60°C under nitrogen protection, reacted for 40 minutes, cooled to room temperature after the reaction was completed, and separated the catalyst to obtain the highly reactive D2 structure hexafluoropropylene dimerization ...
Embodiment 2
[0033] A highly reactive D2 structure hexafluoropropylene dimer is prepared according to the following method:
[0034] Add 0.08Kg of oligomerization catalyst, 0.05Kg of structural isomerization aid and 60Kg of organic solvent into the reaction kettle, stir and mix evenly, use argon to bubble for 8 minutes, then vacuumize, fill with argon for 3 times, under vacuum condition Charge 21Kg of hexafluoropropylene at the bottom, control the temperature at 35°C, stir and react for 5 hours, after completing the reaction, cool down to 8°C, then discharge the residual hexafluoropropylene gas, separate the liquid to obtain the lower layer liquid, add it to the autoclave, and add 0.06Kg of metal Fluoride and 0.25Kg structural isomerization additive, heated to 70°C under nitrogen protection, reacted for 50 minutes, cooled to room temperature after the reaction was completed, and separated the catalyst to obtain the highly reactive D2 structure hexafluoropropylene dimer body.
[0035] The ...
Embodiment 3
[0042] A highly reactive D2 structure hexafluoropropylene dimer is prepared according to the following method:
[0043] Add 0.15Kg of oligomerization catalyst, 0.1Kg of structural isomerization aid and 70Kg of organic solvent into the reaction kettle, stir and mix evenly, use argon to bubble for 10 minutes, then vacuumize, fill with argon for 3 times, and Charge 28Kg of hexafluoropropylene at the bottom, control the temperature at 45°C, stir and react for 8 hours, after completing the reaction, cool down to 10°C, then discharge the residual hexafluoropropylene gas, separate the liquid to obtain the lower layer liquid, add it to the high-pressure reactor, and add 0.12Kg of metal Fluoride and 0.5Kg structural isomerization aid, heated to 80°C under nitrogen protection, reacted for 60 minutes, cooled to room temperature after the reaction was completed, and separated the catalyst to obtain the highly reactive D2 structure hexafluoropropylene dimerization body.
[0044] The prepa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com