A kind of β-fluoroalkyl cinnamate compound and preparation method thereof
A technology for cinnamate compounds and compounds is applied in the field of beta-fluoroalkyl cinnamate compounds and their preparation, which can solve the problems of side reactions, less selectivity and the like, and achieves the advantages of less side products, simple operation and easy preparation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
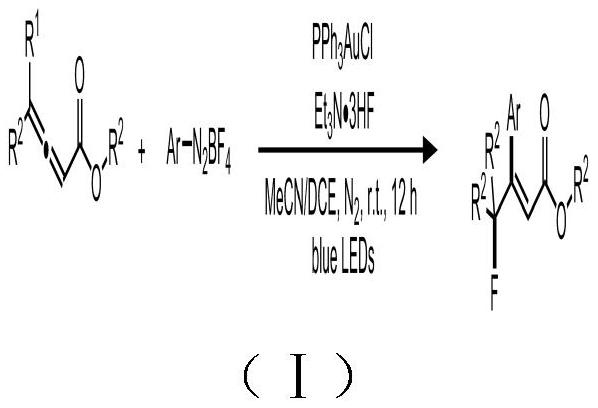
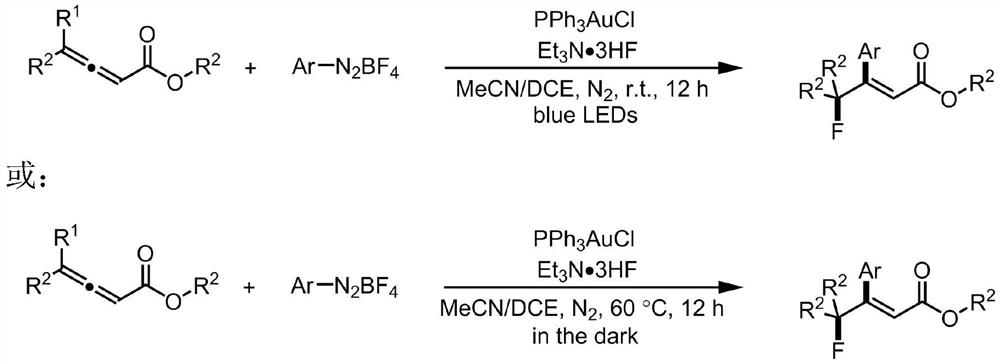
[0030] (1) Under nitrogen atmosphere, mix 0.1mmol benzyl 4-phenyl allenoate, 0.2mmol tetrafluoroborate 4-ethoxycarbonylphenyl diazonium salt, 1.0mmol triethylamine trihydrofluoride, 0.02mmol Ph 3 PAuCl was sequentially added to a reaction tube containing a mixed solvent of 0.3mL acetonitrile and 0.2mL 1,1-dichloroethane;
[0031] (2) Under nitrogen protection and room temperature, the reaction tube in which the reaction mixture was dissolved was irradiated under the blue light of 15W LED for 12 hours, and the reaction solution was filled with a column of 200-300 mesh silica gel, using ethyl acetate and The eluent with a volume ratio of petroleum ether of 10:90 was subjected to column separation to obtain pure β-fluoroalkyl cinnamate compound 1.
Embodiment 2
[0033] (1) Under nitrogen atmosphere, add 0.1mmol 4-phenyl allenoic acid phenyl ester, 0.2mmol tetrafluoroborate 4-ethoxycarbonylphenyl diazonium salt, 1.0mmol triethylamine trihydrofluoride, 0.02mmol Ph 3 PAuCl was sequentially added to a reaction tube containing a mixed solvent of 0.3 mL of acetonitrile and 0.2 mL of 1,1-dichloroethane.
[0034] (2) Under the protection of nitrogen and at room temperature, the reaction tube in which the reaction mixture was dissolved was irradiated under the blue light of 15W LED for 12 hours, and the reaction solution was filled with a column of 200-300 mesh silica gel, using ethyl acetate and petroleum The eluent with ether volume ratio of 10:90 was subjected to column separation to obtain β-fluoroalkyl cinnamate compound 2.
Embodiment 3
[0036] (1) Under nitrogen atmosphere, add 0.1mmol ethyl 4-phenyl allenoate, 0.2mmol tetrafluoroborate 4-ethoxycarbonylphenyl diazonium salt, 1.0mmol triethylamine trihydrofluoride, 0.02mmol Ph 3 PAuCl was sequentially added to a reaction tube containing a mixed solvent of 0.3 mL of acetonitrile and 0.2 mL of 1,1-dichloroethane.
[0037] (2) Under nitrogen protection and room temperature, the reaction tube in which the reaction mixture was dissolved was irradiated under the blue light of 15W LED for 12 hours, and the reaction solution was filled with a column of 200-300 mesh silica gel, using ethyl acetate and The eluent with a volume ratio of petroleum ether of 10:90 was subjected to column separation to obtain β-fluoroalkyl cinnamate compound 3.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com