Interleukin 11 mutant and application thereof in treating hepatic fibrosis
A mutant and human interleukin technology, applied in the field of biomedicine, can solve problems such as the absence of satisfactory drugs, and achieve the effect of inhibiting the activation of signal pathways, inhibiting activation, and broad medicinal prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the construction of interleukin 11 mutant
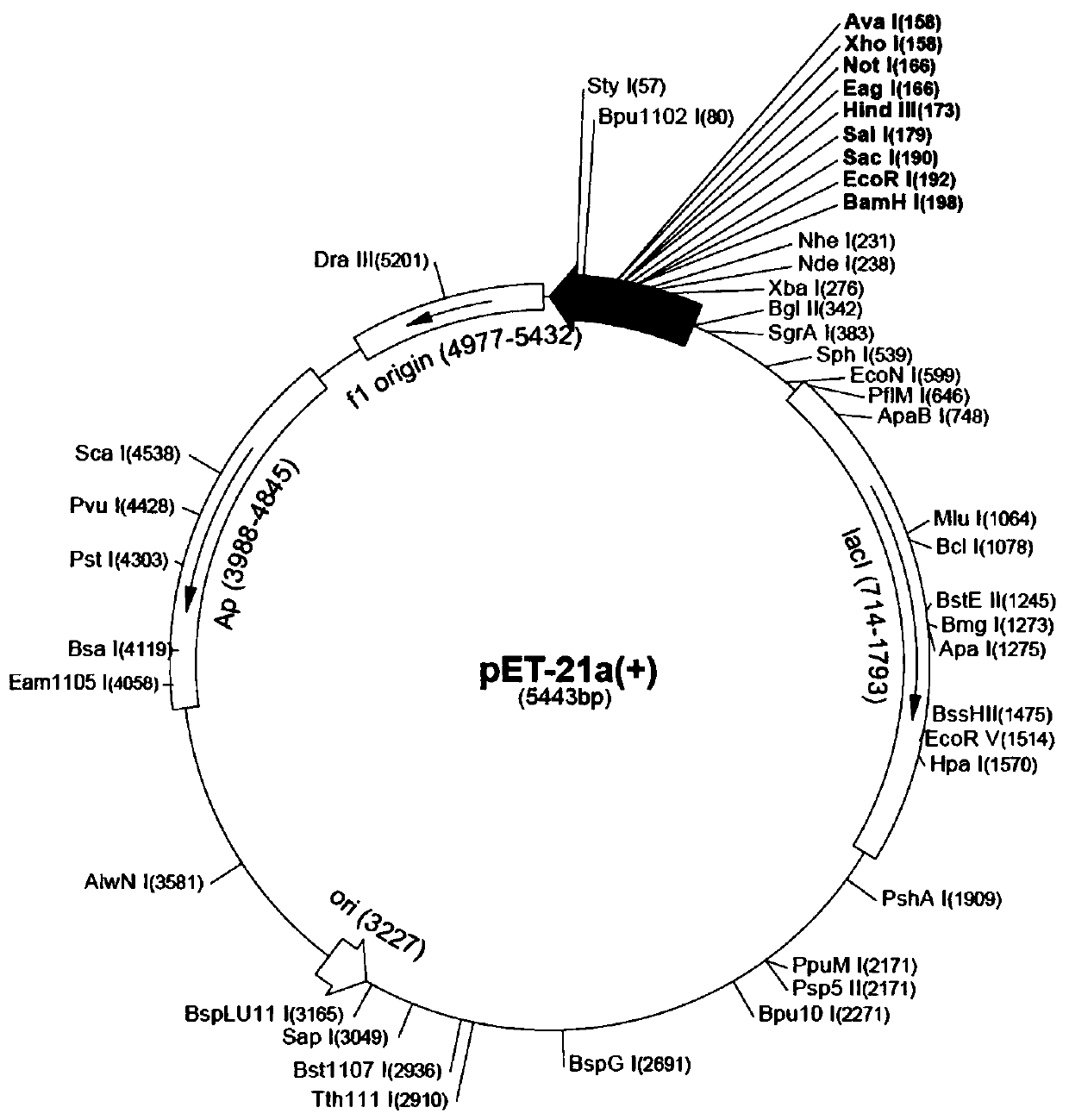
[0029]pET-21a was selected as the construction vector of IL-11 and its mutants. According to the cDNA sequence of IL-11, the following primers were used for PCR amplification: FP: 5'- CGCGGATCCGACTACAAAGACGATGACGACAAGCCTGGGCCACCACCTGGC-3', the upstream primer was inserted into the BamH I restriction site; RP: 5'-CCGGAATTCTCACAGCC GAGTCTTCAGCAGCAGCAGTCC-3', the downstream primer was inserted EcoR I restriction site. A Flag tag was introduced at the N-terminus during primer design for subsequent protein purification and detection. The amplified IL-11 gene fragment was digested together with the pET-21a plasmid vector, T4 DNA ligase and its Buffer were added to the digested product, and allowed to stand at room temperature for 10 minutes. The ligation product was added to Escherichia coli DH5α competent for heat shock transformation. Put it into a constant temperature shaker to activate the amplification, and the...
Embodiment 2
[0030] Example 2: Expression and purification of interleukin-11 and its mutants
[0031] The interleukin-11 plasmid pET-21a-hIL11 and its mutant plasmids in Example 1 were transformed into Escherichia coli BL21 (DE3) competent, placed in a constant temperature shaker for activation, and then inoculated into LB medium containing ampicillin resistance, After culturing at 37°C for 3 hours, the OD value was kept at 0.4-0.6, and then 1 mM IPTG was added for induction for 4 hours. Collect the thalline, lyse the thalline with the bacterial lysate (50mM HEPES buffer contains 0.1% Triton X-100 and 150ug / ml lysozyme, pH=7.4) for 30min, and the bacterial lysate is ultrasonically broken to a non-viscous state, Then centrifuge at 13,000 rpm for 20 min at 4°C, discard the precipitate, and repeat twice. To concentrate the crude protein, add the supernatant to a saturation of 60% (NH4) 2 SO 4 , to obtain concentrated crude protein. The salt was removed by dialysis with 50 mM HEPES buffer ...
Embodiment 3
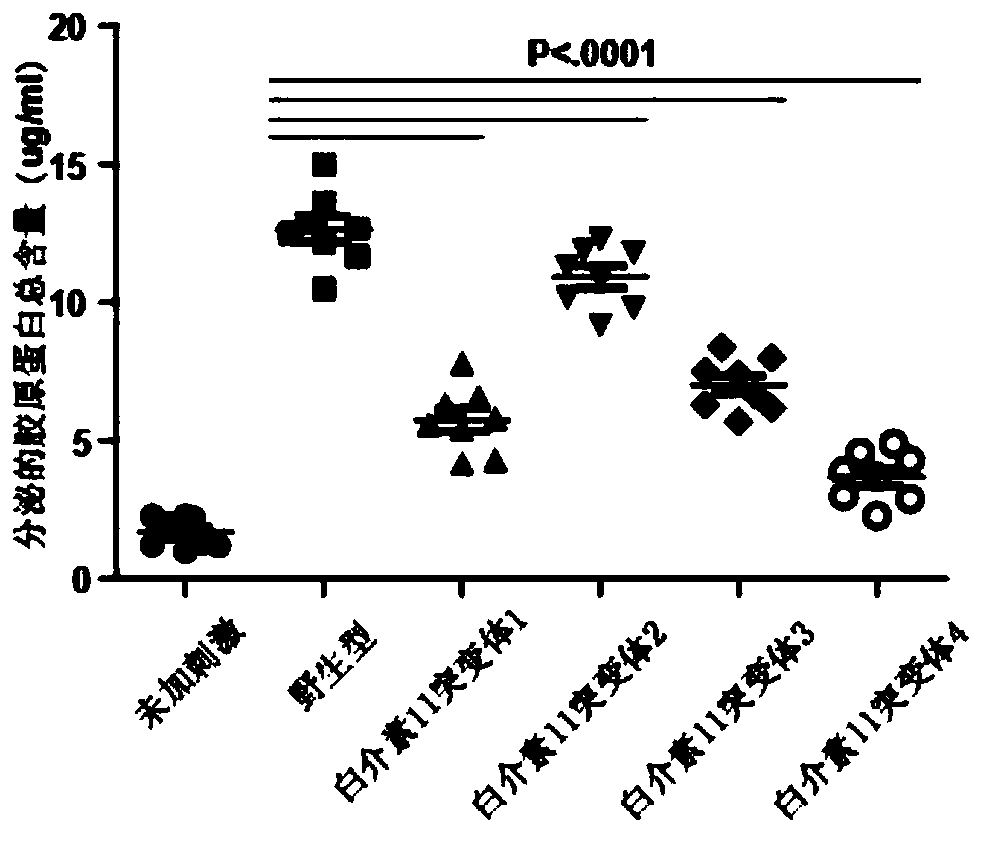
[0032] Example 3: Affinity detection of interleukin-11 mutants
[0033] The binding affinity of IL11 obtained in Example 2 and its mutants at 50% effective concentration (EC50) was evaluated by ELISA experiment. 0.8 μg / mL of recombinant human IL11Rα was coated on the Elisa plate, overnight at 4°C, and the sites not bound to IL11Rα were blocked with 2% BSA. IL11 and its mutants were diluted with PBS containing 1% BSA to a concentration gradient of 1000ng / ml, 250ng / ml, 62.5ng / ml, 15.63ng / ml, 3.91ng / ml, 0.98ng / ml. The samples of IL11 and its mutants were sequentially added to the ELISA plate according to the concentration gradient, sealed with a sealing film, and incubated at room temperature for 2 hours. After washing the plate, the detection antibody Flag-HRP was added, sealed with a sealing membrane, and incubated at room temperature for 2 h. Add washing buffer, stay for 1min, discard the liquid, tap dry, repeat 3 times to wash away the unbound detection antibody. Add TMB c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com