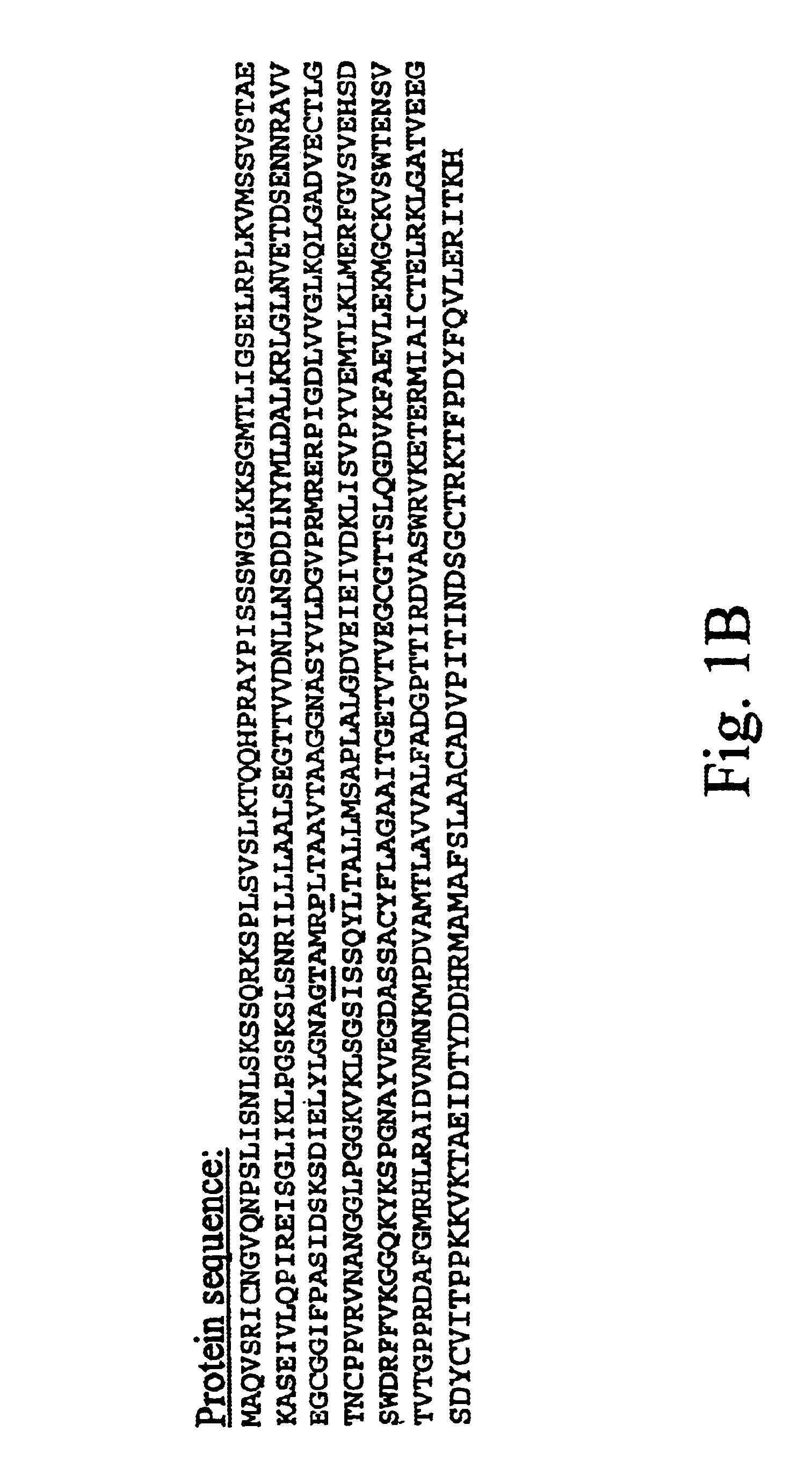
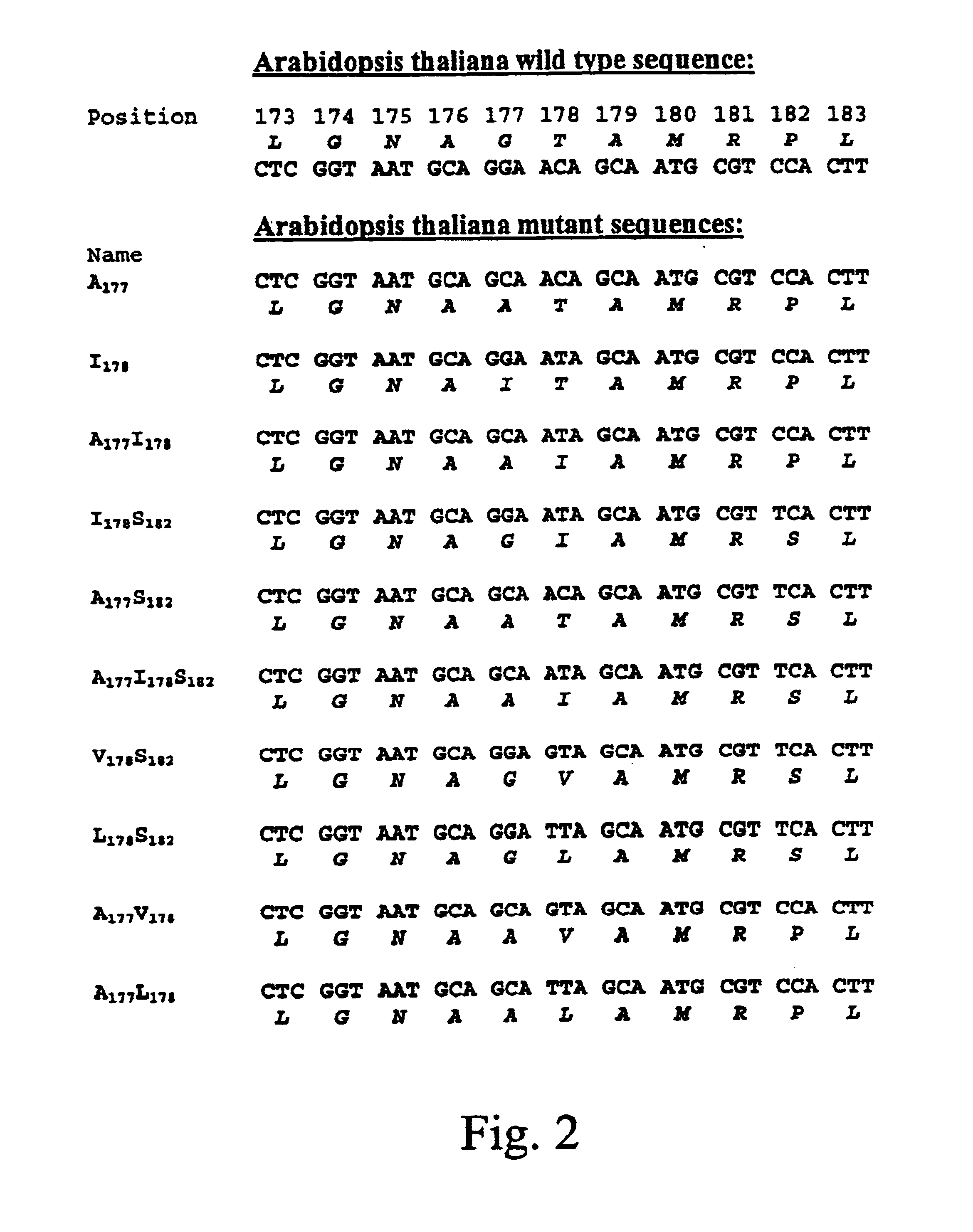
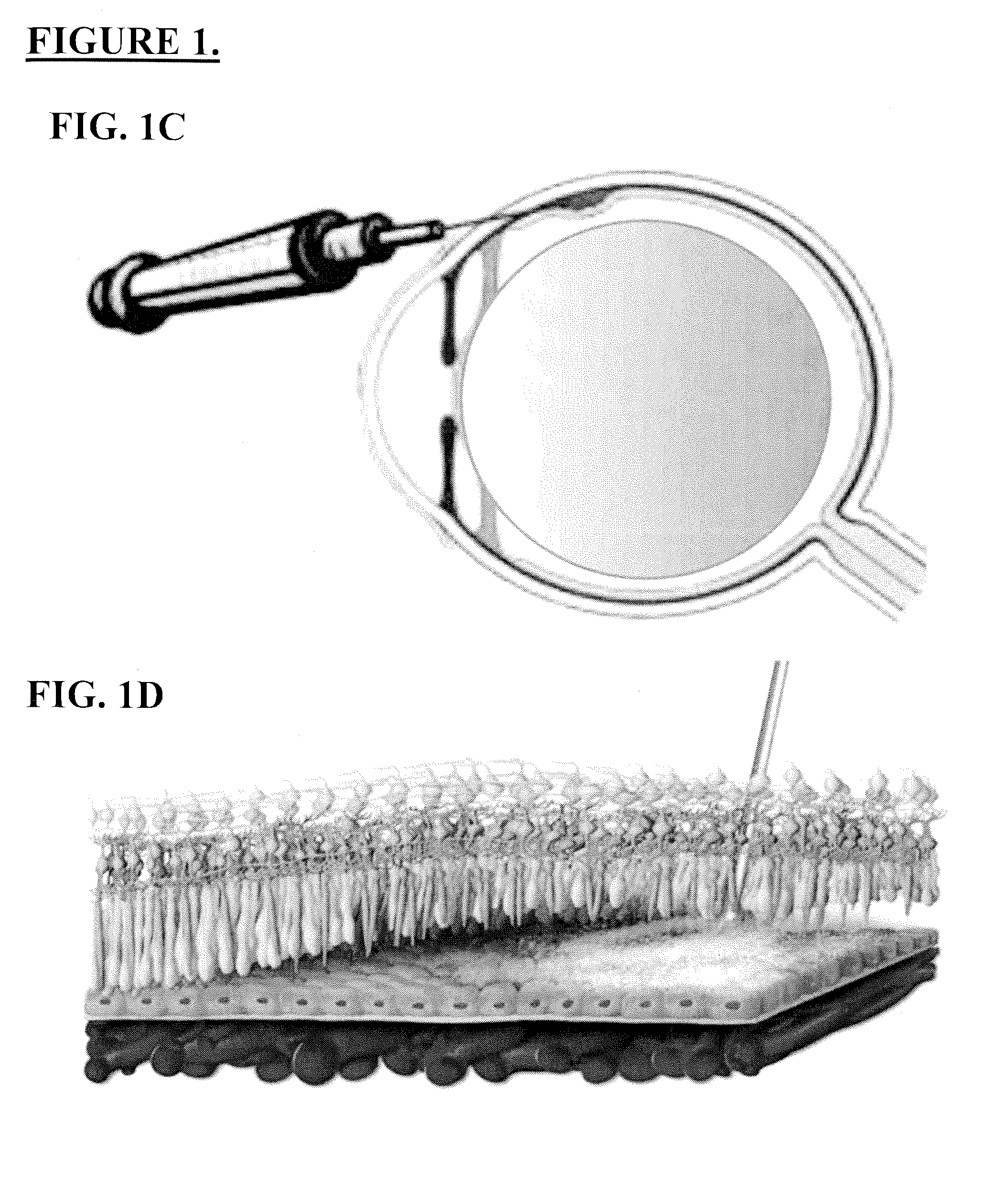
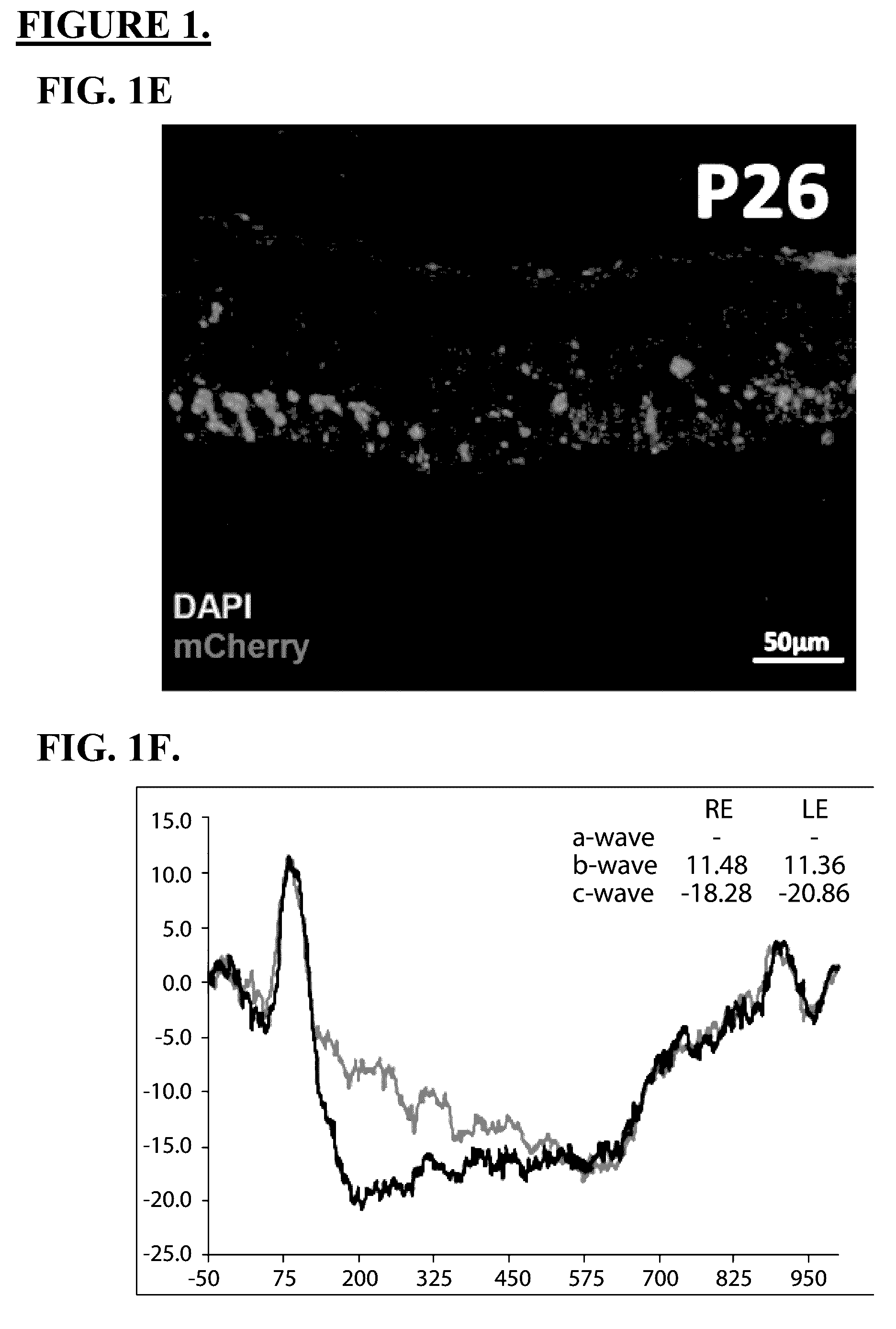
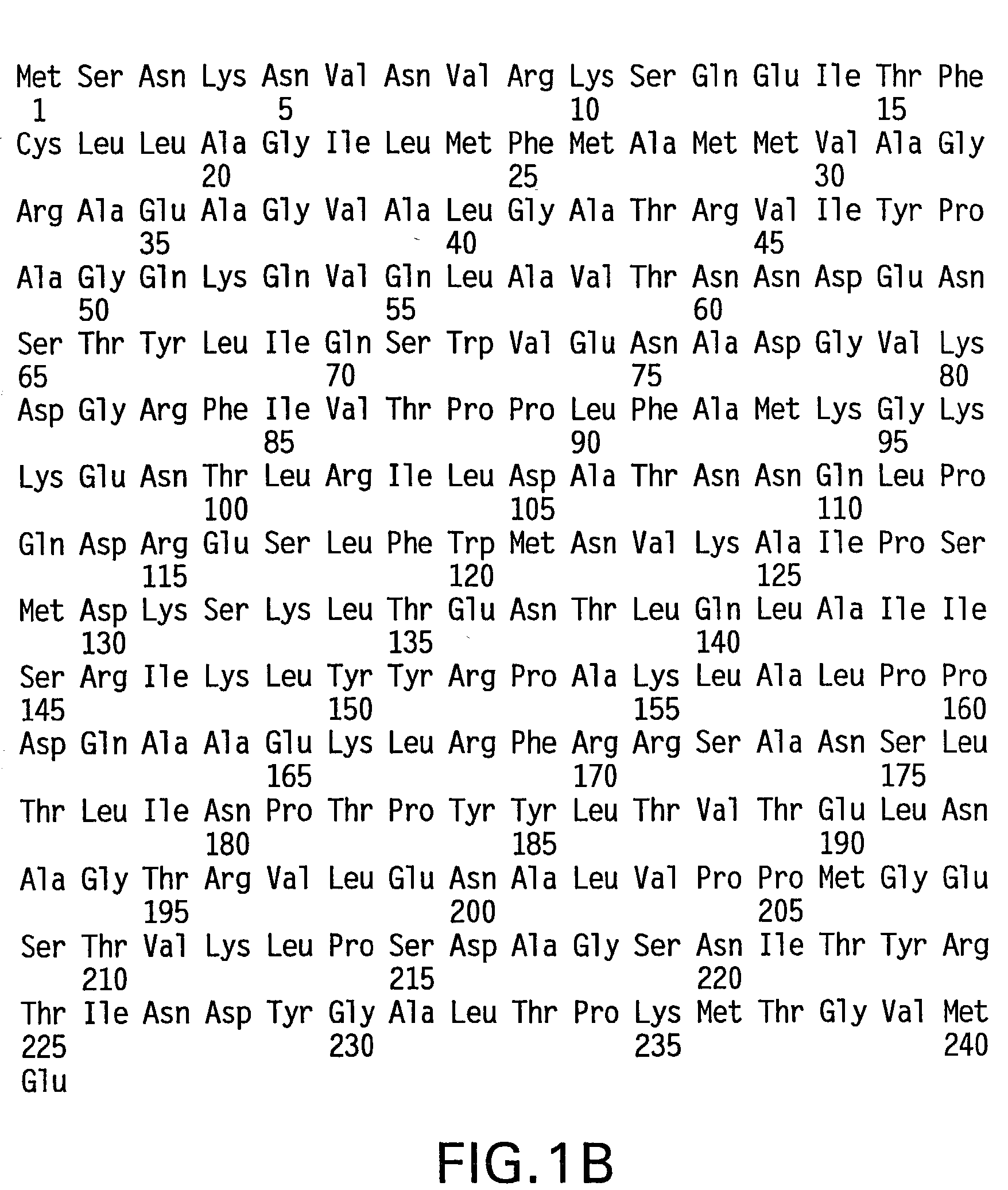Patents
Literature
217 results about "Wild type protein" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
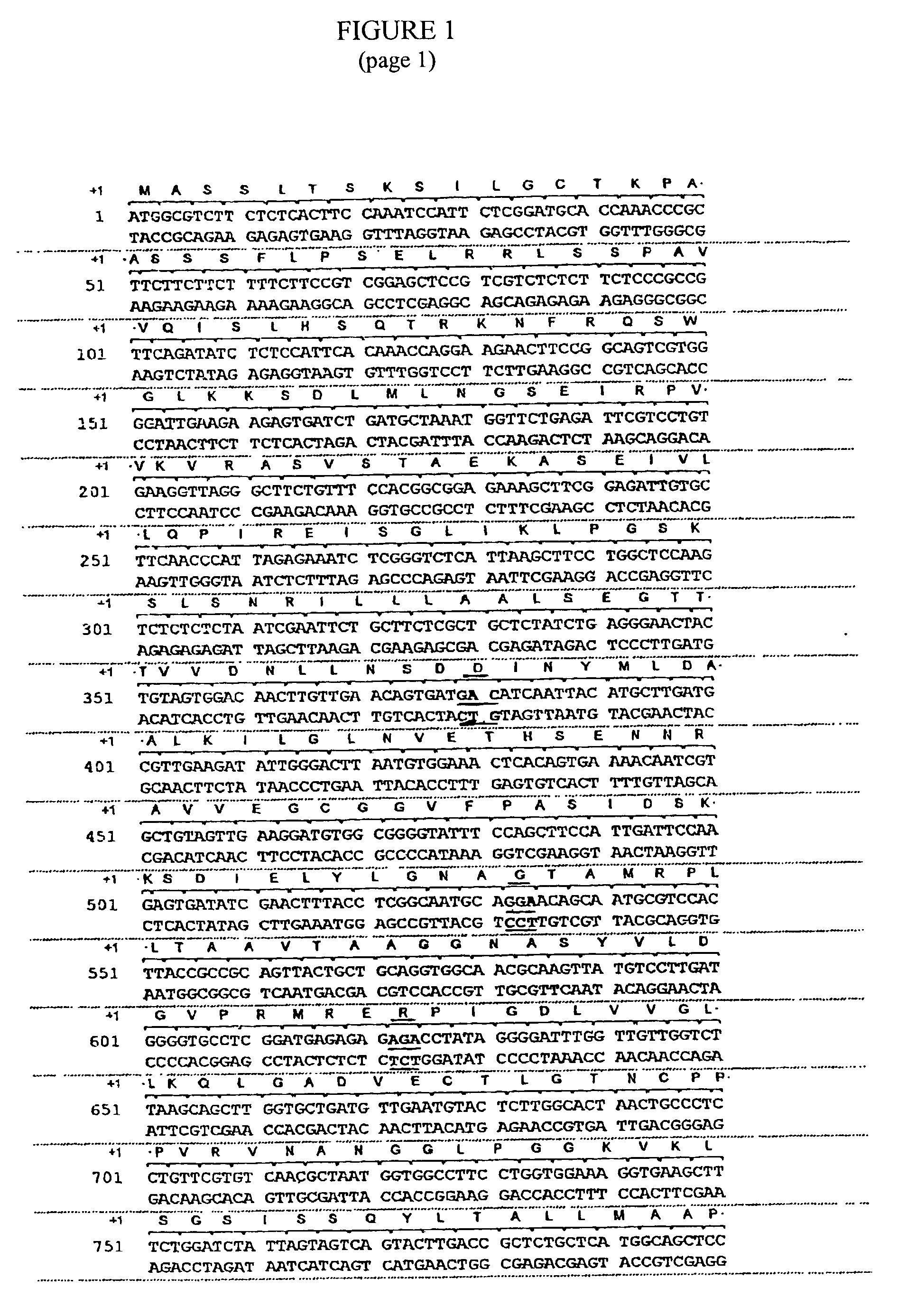
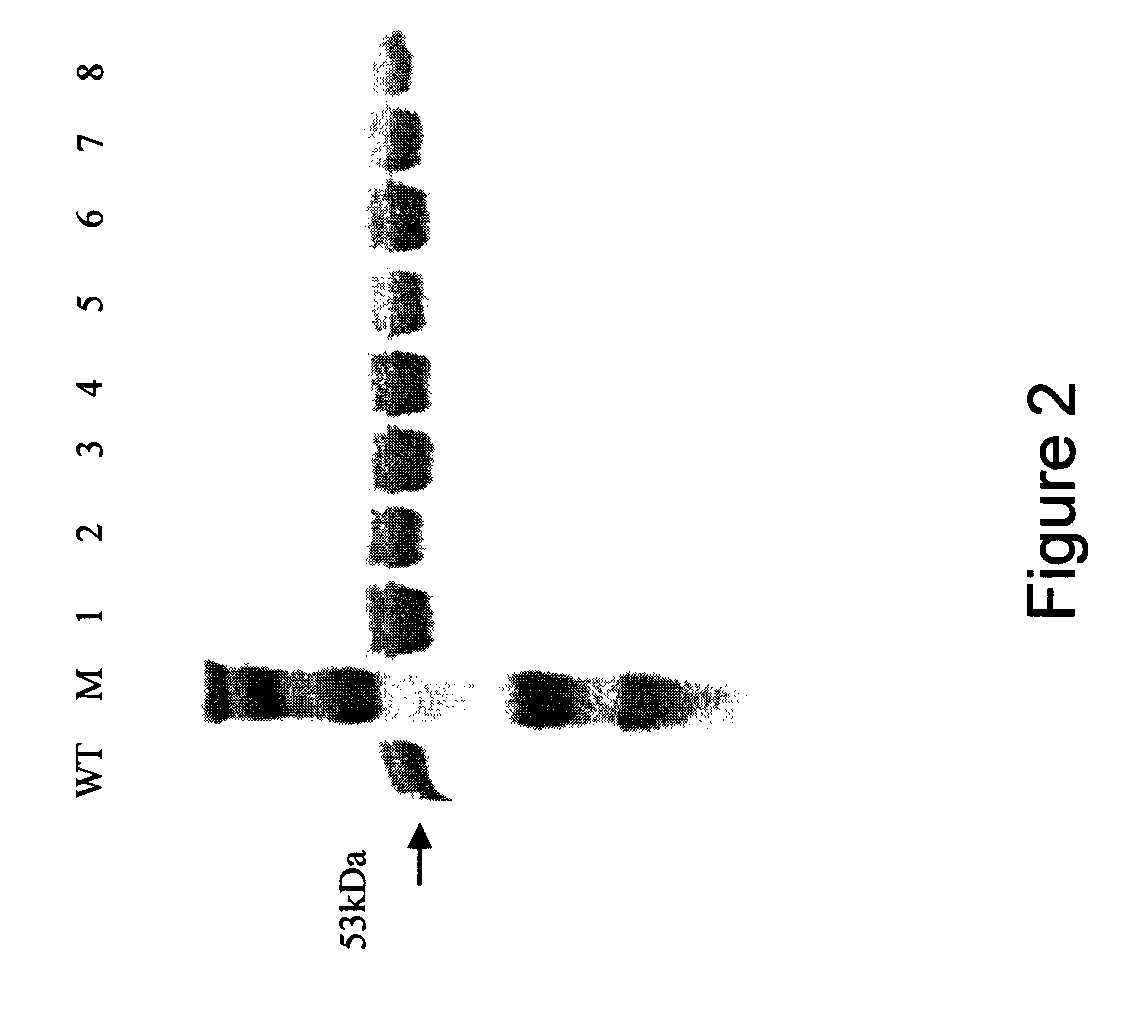
The wild-type human K-Ras4B protein has been produced in a bacterial expression system. The recombinant protein contains six histidine residues at its amino terminus (His-tag).
Methods of making non-transgenic herbicide resistant plants
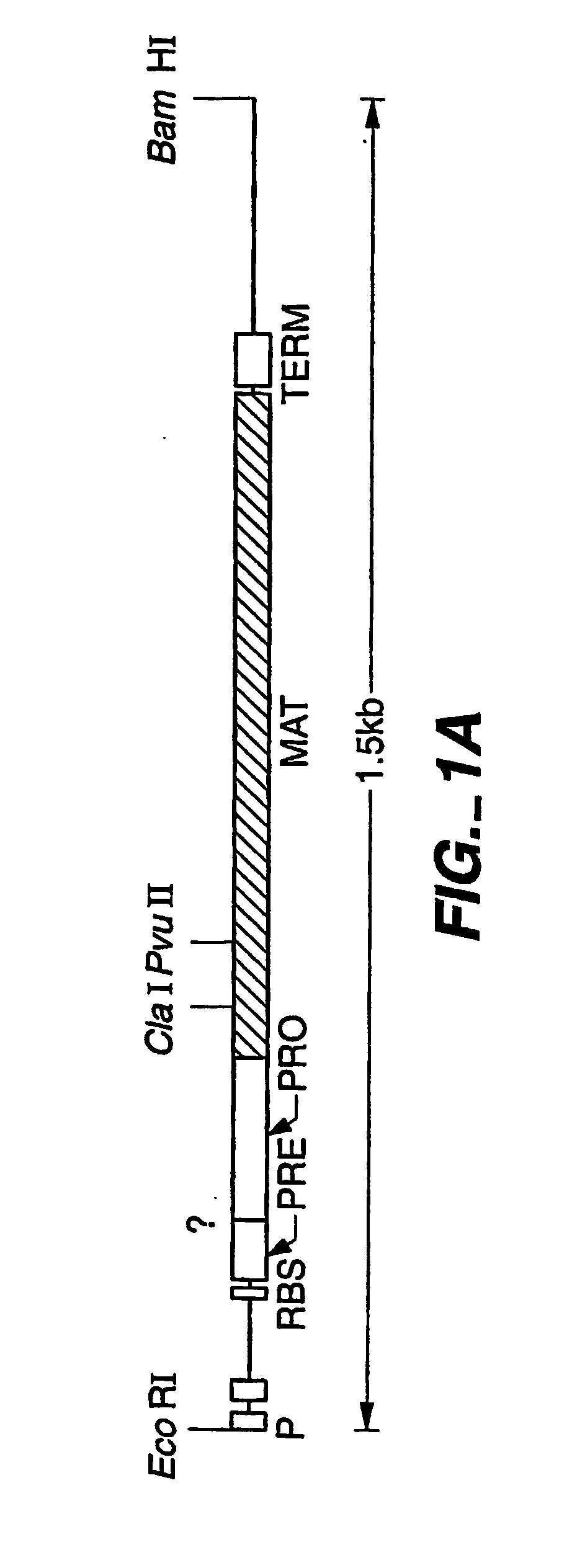
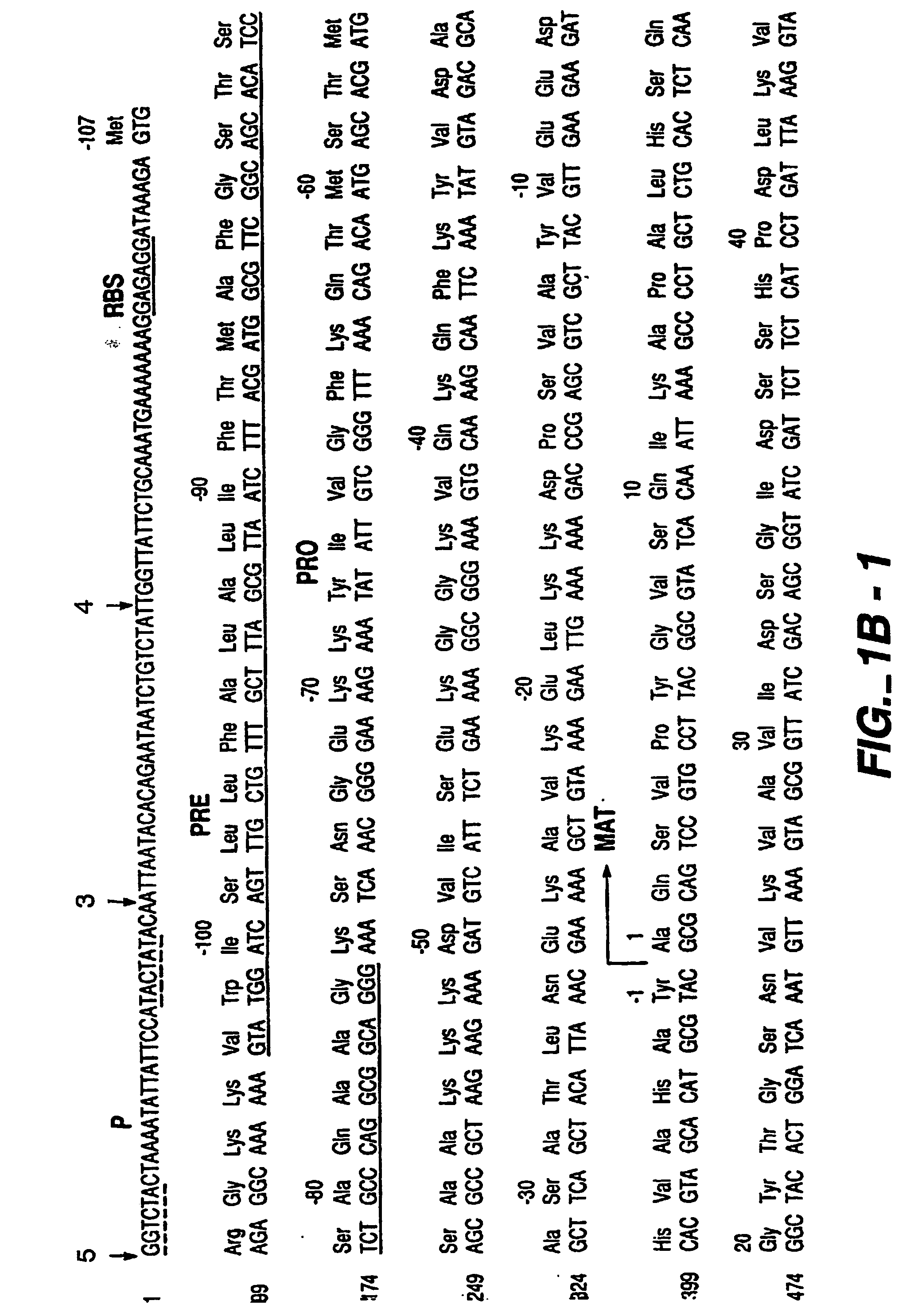
The present invention relates to the production of a non-transgenic plant resistant or tolerant to a herbicide of the phosphonomethylglycine family, e.g., glyphosate. The present invention also relates to the use of a recombinagenic oligonucleobase to make a desired mutation in the chromosomal or episomal sequences of a plant in the gene encoding for 5-enol pyruvylshikimate-3-phosphate synthase (EPSPS). The mutated protein, which substantially maintains the catalytic activity of the wild-type protein, allows for increased resistance or tolerance of the plant to a herbicide of the phosphonomethylglycine family, and allows for the substantially normal growth or development of the plant, its organs, tissues or cells as compared to the wild-type plant irrespective of the presence or absence of the herbicide.
Owner:CIBUS
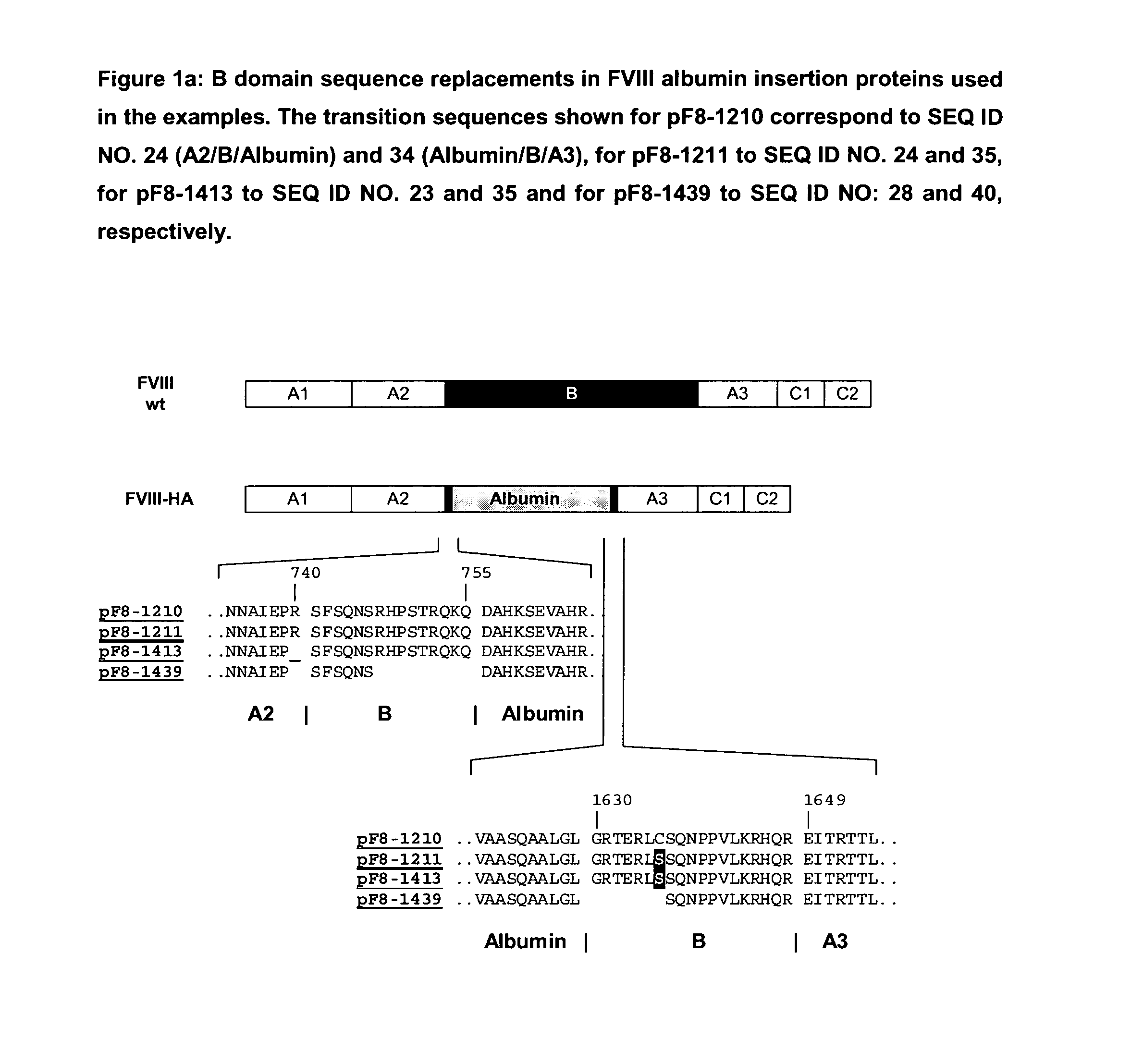
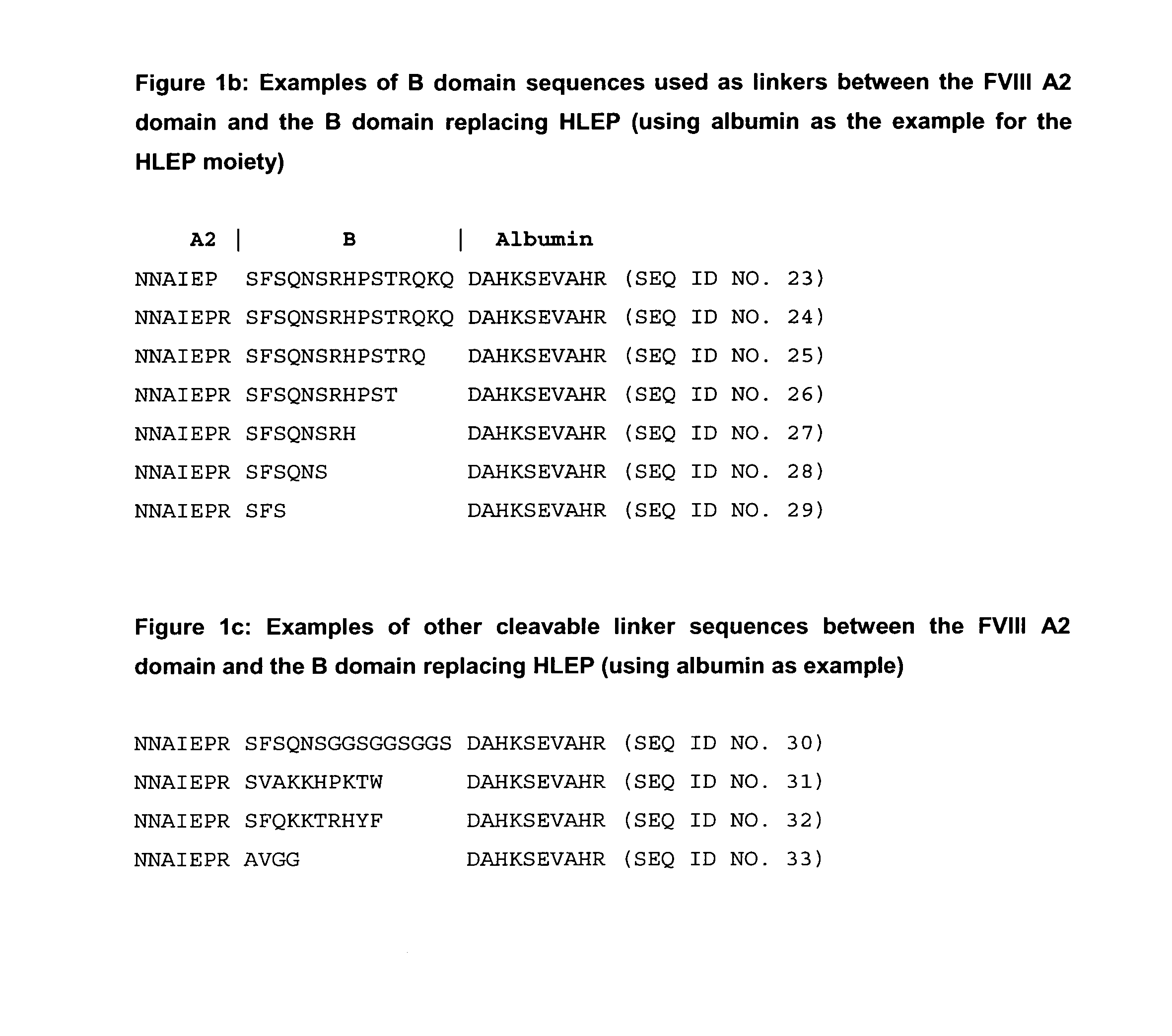
Modified coagulation factors with prolonged in vivo half-life
InactiveUS20100120664A1Increase productionHigh expressionFactor VIIPeptide/protein ingredientsHalf-lifeNucleic acid sequencing
The present invention relates to nucleic acid sequences coding for modified coagulation factors, preferably coagulation factor VIII, and their derivatives; recombinant expression vectors containing such nucleic acid sequences; host cells transformed with such recombinant expression vectors; and recombinant polypeptides and derivatives coded for by said nucleic acid sequences, whereby said recombinant polypeptides and derivatives have biological activities and prolonged in vivo half-lives compared to the unmodified wild-type proteins. The invention also relates to corresponding sequences that result in improved in vitro stability. The present invention further relates to processes for the manufacture of such recombinant proteins and their derivatives. The invention also relates to a transfer vector for use in human gene therapy, which comprises such nucleic acid sequences.
Owner:CSL BEHRING GMBH
Mirac proteins
This disclosure relates to a method of generating conditionally active biologic proteins from wild type proteins, in particular therapeutic proteins, which are reversibly or irreversibly inactivated at the wild type normal physiological conditions. For example, evolved proteins are virtually inactive at body temperature, but are active at lower temperatures.
Owner:BIOATLA LLC
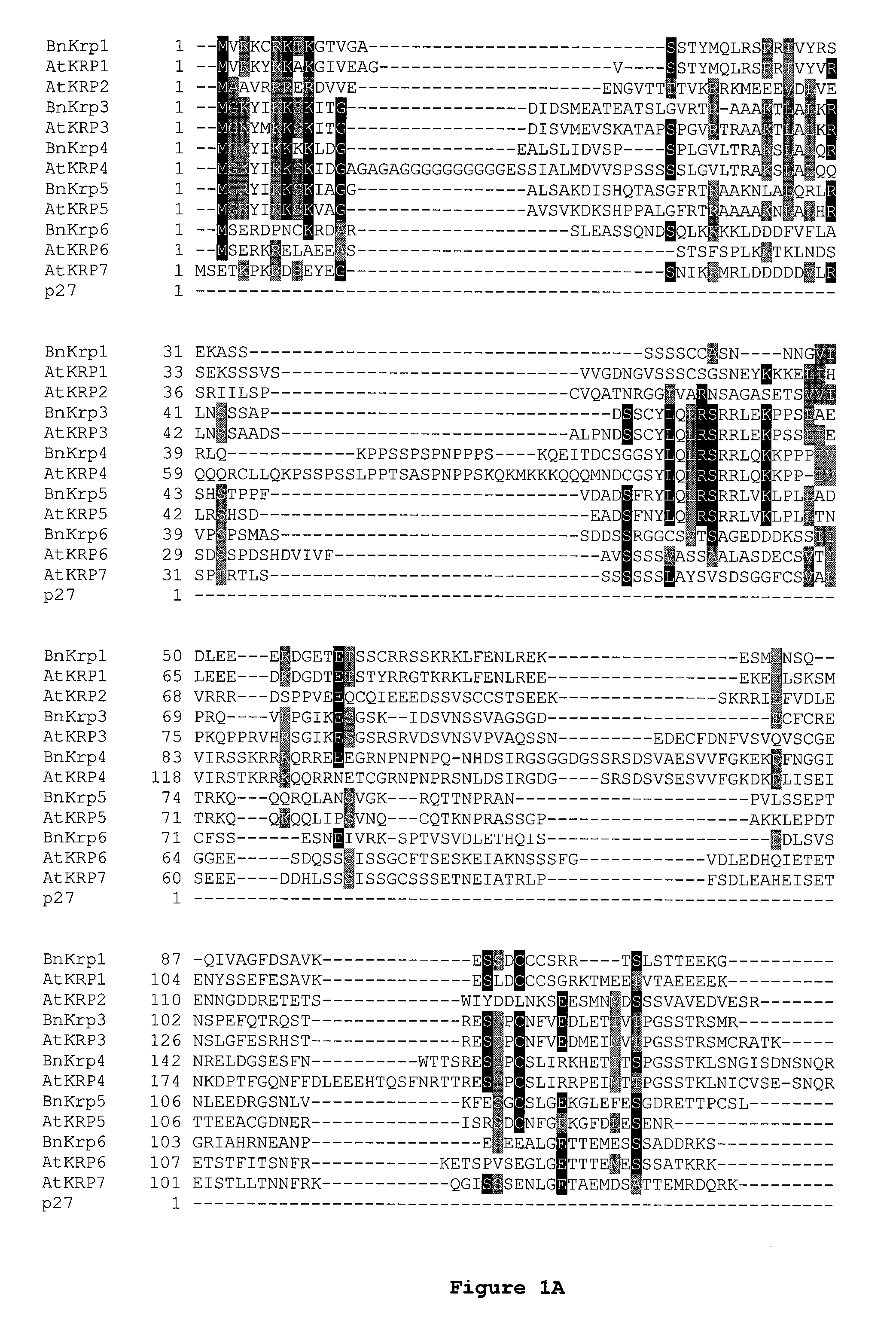
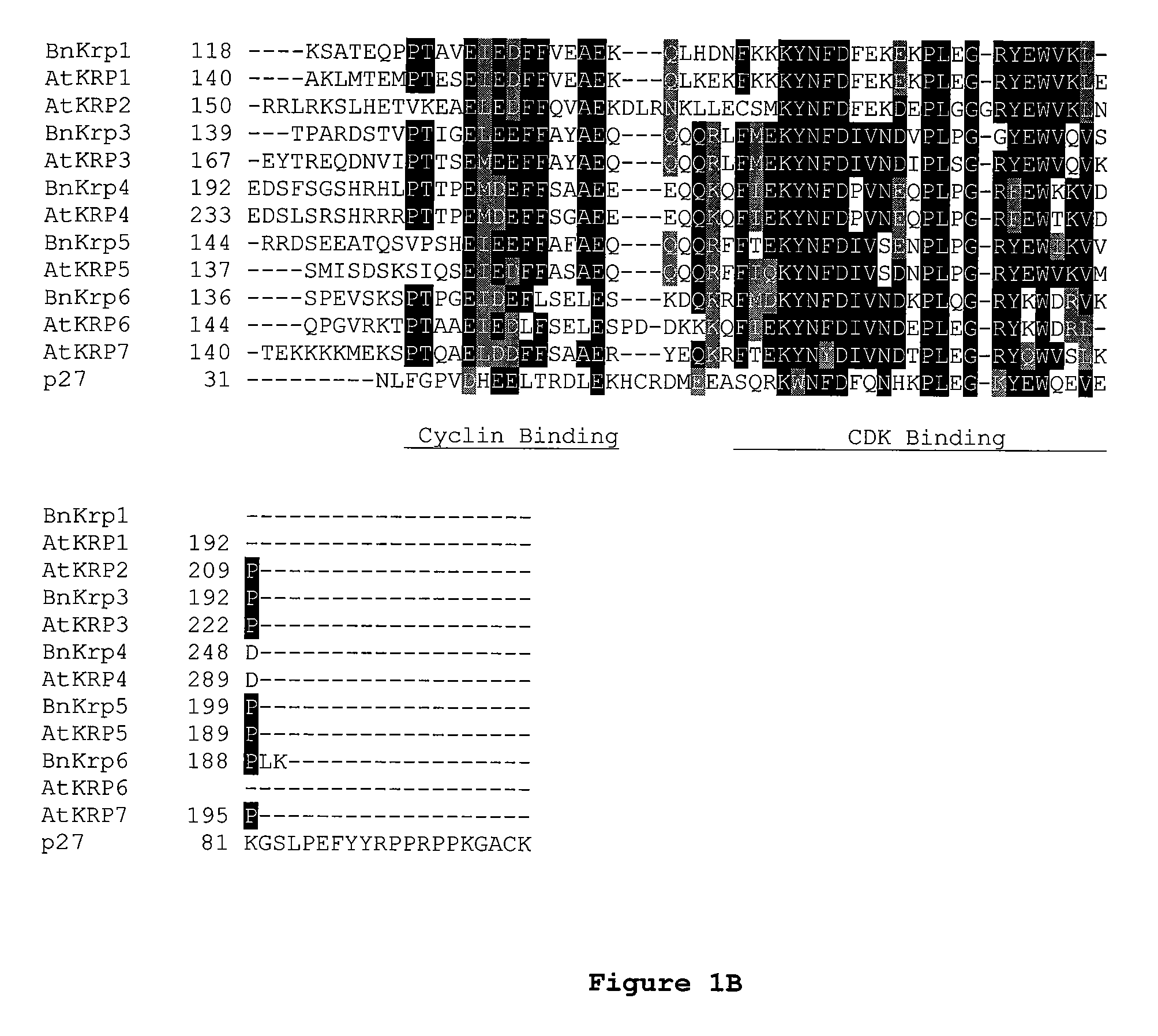
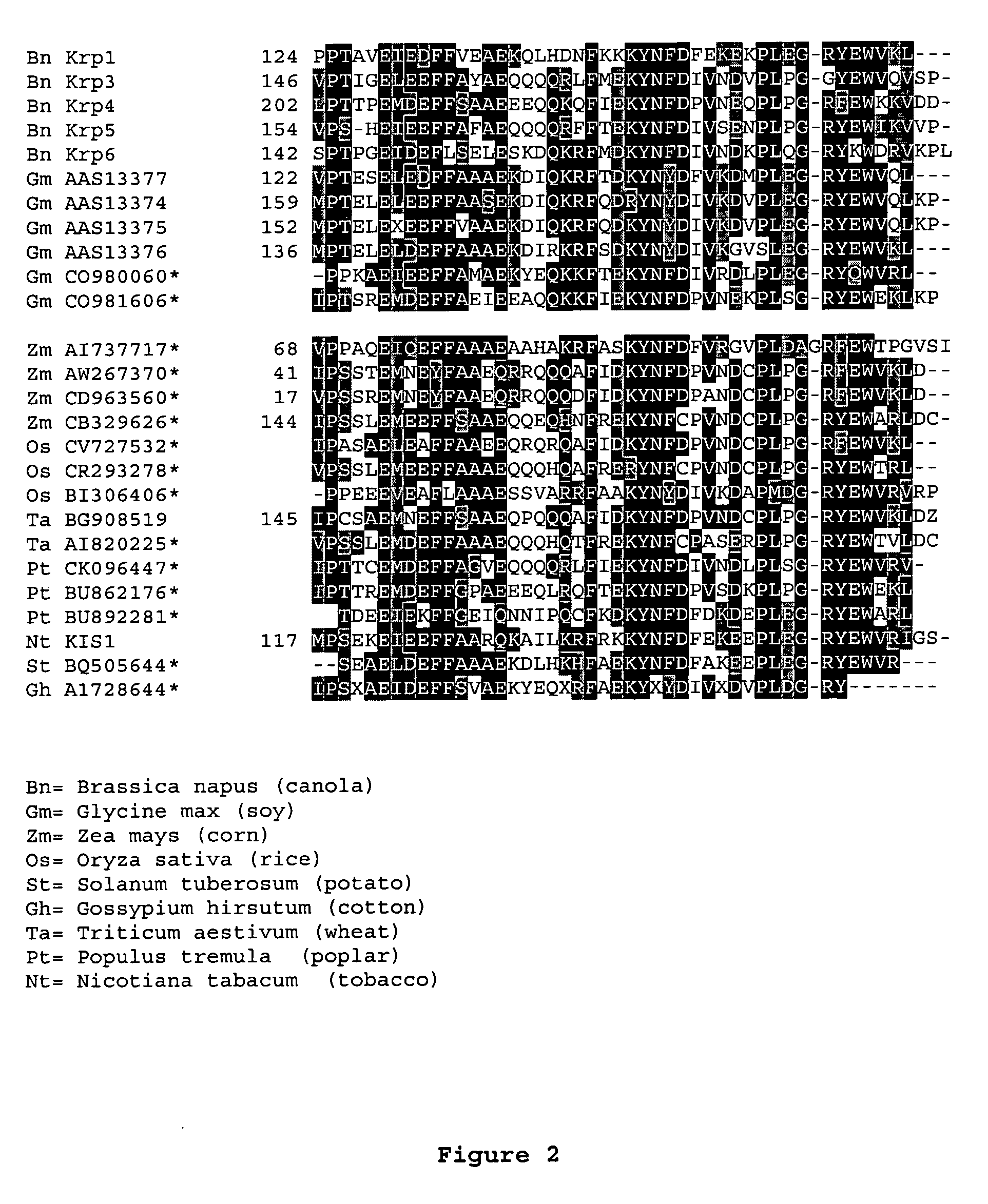
Dominant negative mutant krp protein protection of active cyclin-cdk complex inhibition by wild-type krp
InactiveUS20070056058A1Speed upIncreasing cell proliferationBacteriaSugar derivativesMutated proteinPlant cell
Disclosed are mutant CDK inhibitor (CKI) polypeptides having dominant negative antagonist activity against wild-type CKI proteins, as well as related compositions, including nucleic acids and vectors encoding the mutant CKI polypeptides and transformed host cells and transgenic plants comprising such nucleic acids and vectors. Also disclosed are related methods for using the mutant proteins to modulate cell division in cells, particularly plant cells.
Owner:TARGETED GROWTH
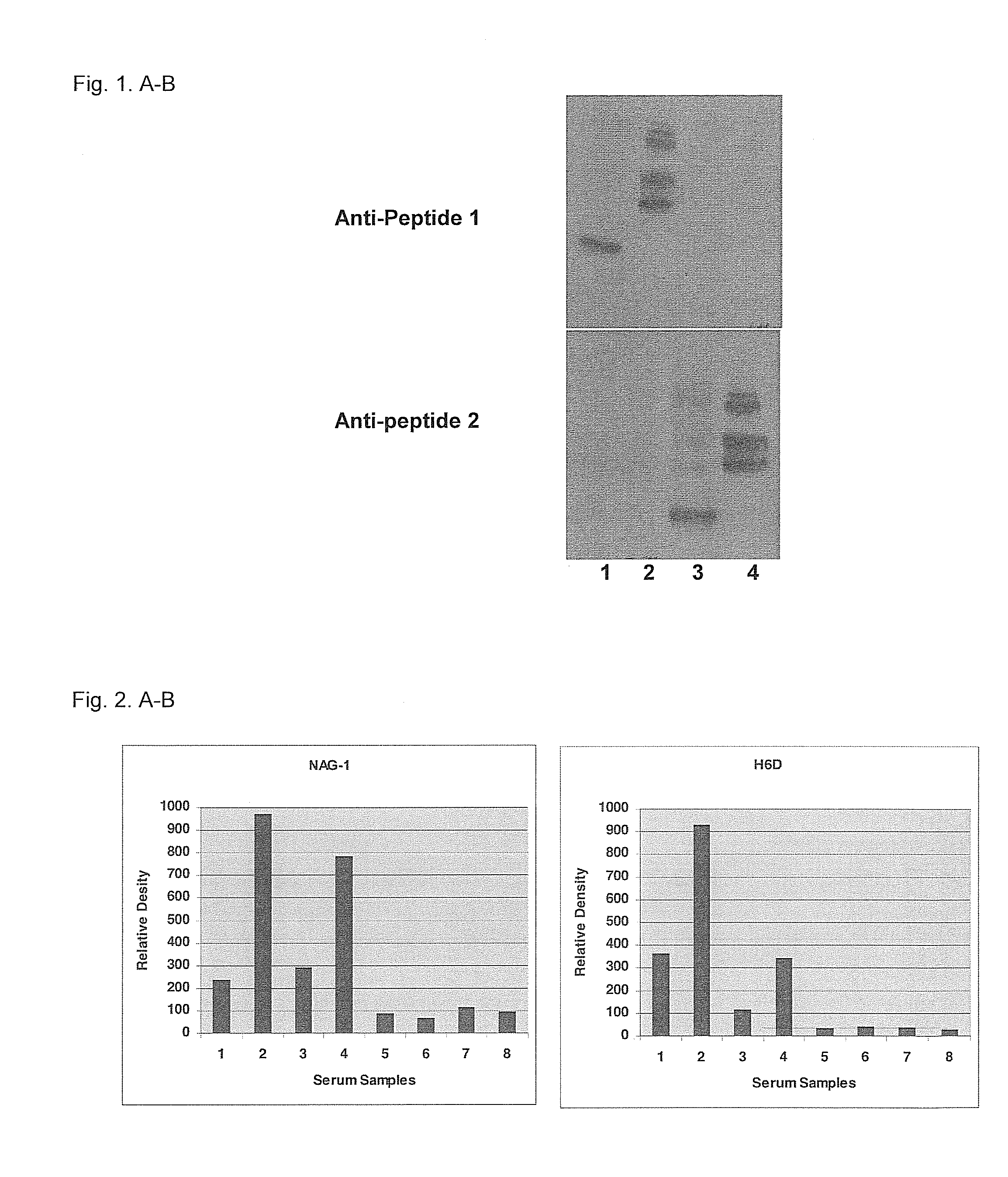
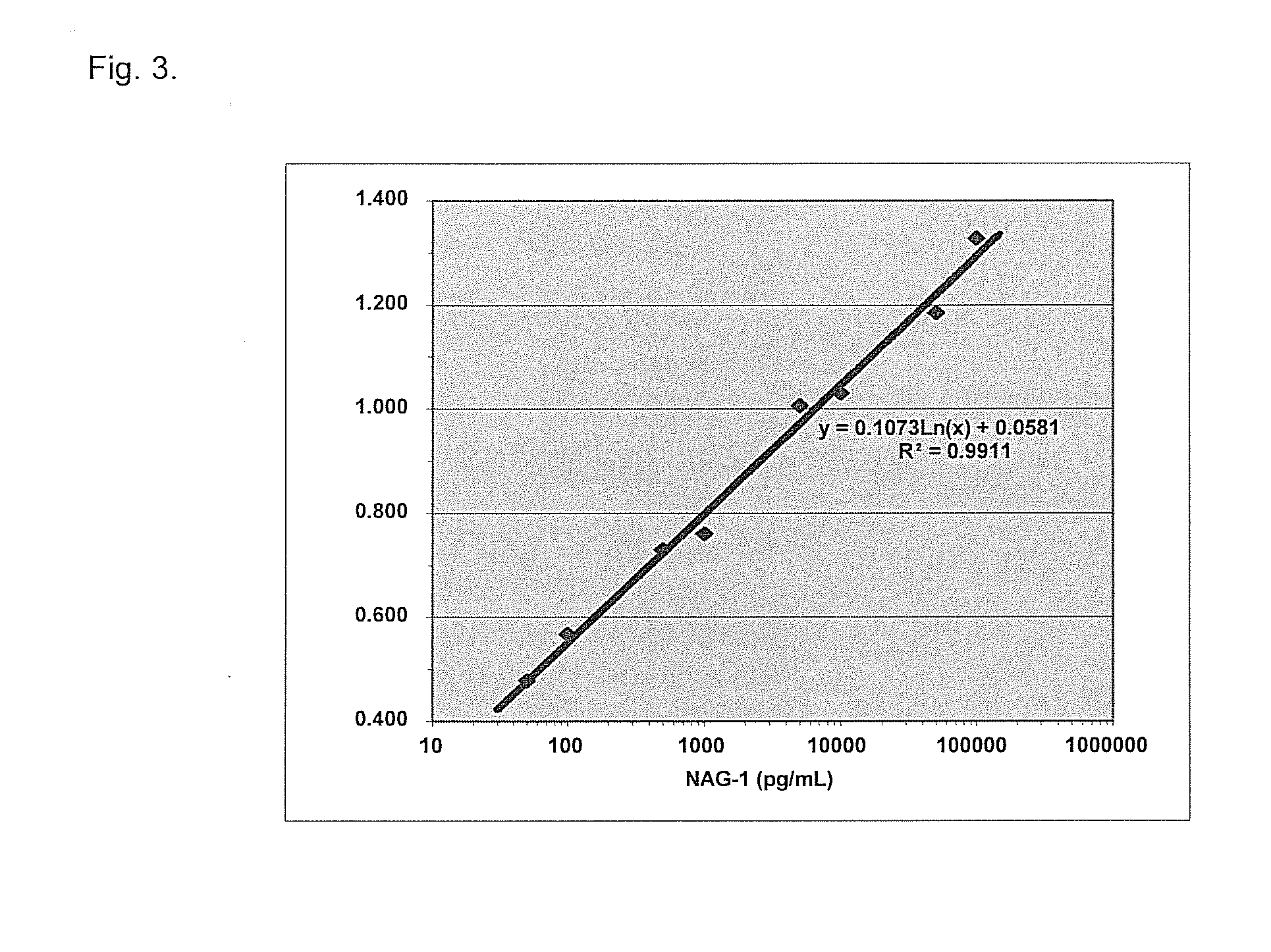
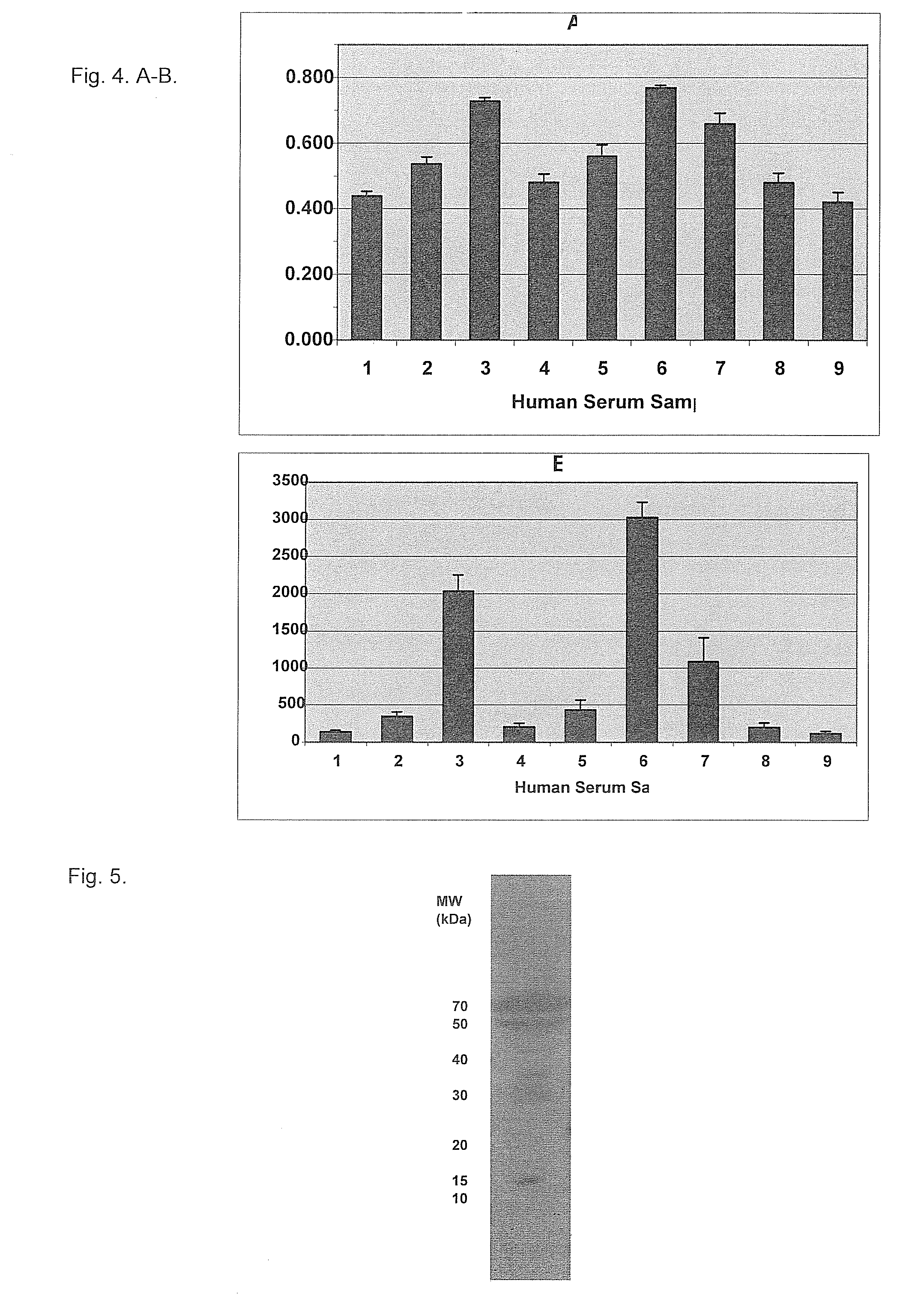
Form-specific antibodies for nag-1 (mic-1, gdf-15), h6d and other tgf-beta subfamily and heart disease and cancer diagnoses
A method of producing form-specific anti-peptide antibodies for a wild type protein and its one amino acid mutated protein using a peptide antigen, by obtaining a protein sequence of the wild type protein and its one amino acid mutated protein, selecting a continuous amino acid sequence without any internal cysteine residues that includes the one amino acid mutated sequence and wild type sequence corresponding to the mutated site at the end of the sequence to obtain a synthetic mutation peptide and a synthetic wild type peptide, conjugating the synthetic peptides to a carrier protein, and immunizing an animal to produce antibodies. Methods of detecting cancer and methods of treating cancer.
Owner:DETROIT R&D
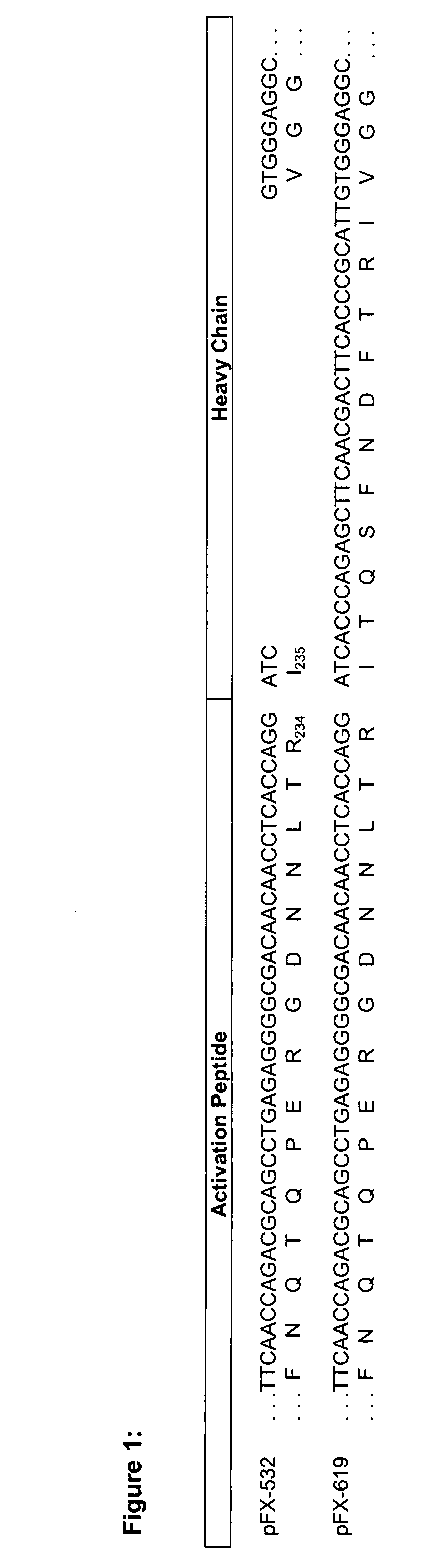
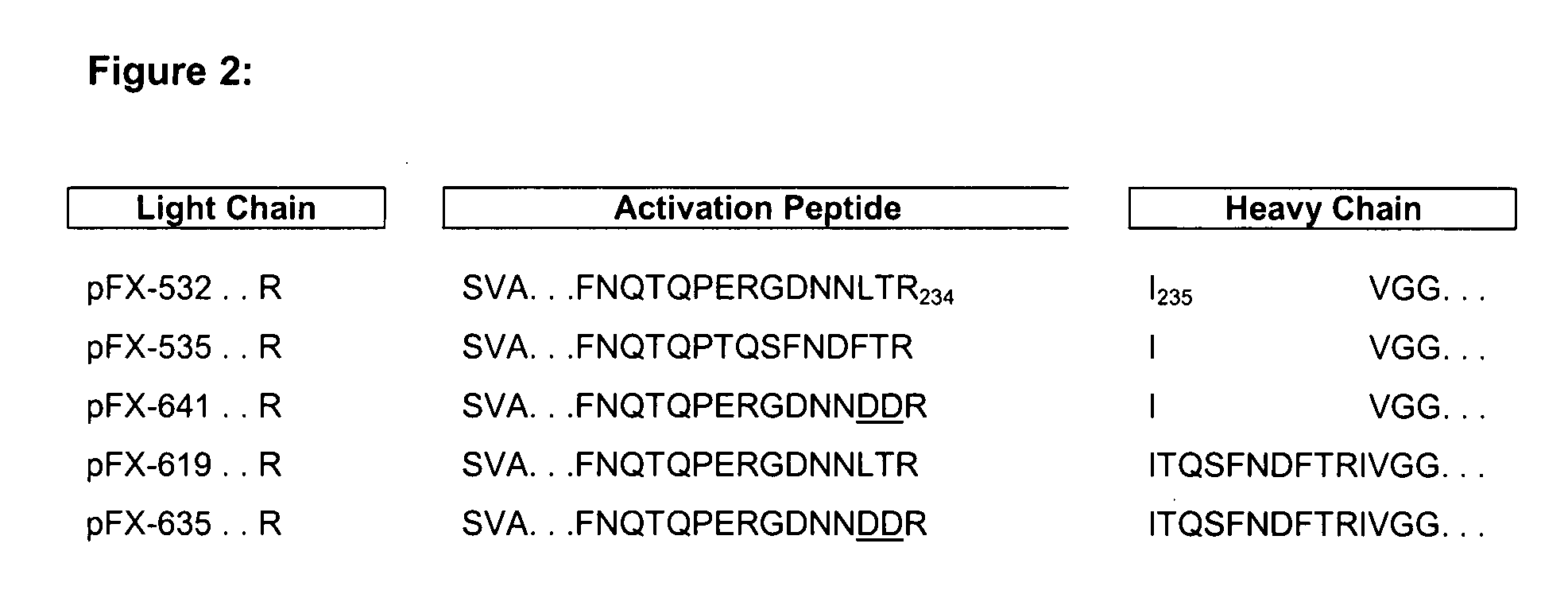
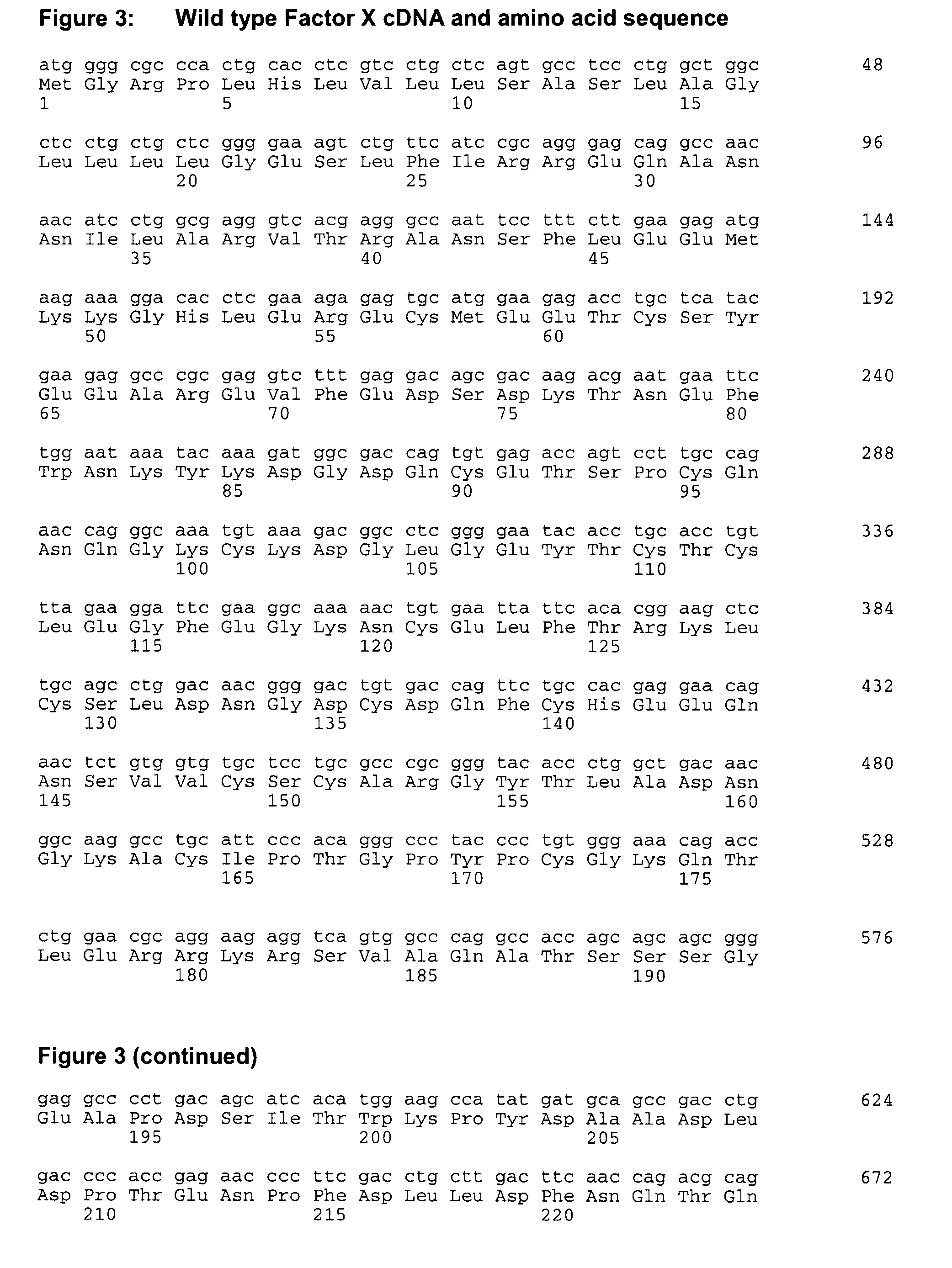
Coagulation factor x polypeptides with modified activation properties
The present invention relates to modified cDNA sequences coding for human Factor X and their derivatives with improved stability and modified activation sequences, recombinant expression vectors containing such cDNA sequences, and host cells transformed with such recombinant expression vectors. The invention also relates to recombinant factor X polypeptides and derivatives which have biological activities of the unmodified wild type protein but with improved stability and processes for the manufacture of such recombinant proteins and their derivatives. The invention also covers a transfer vector for use in human gene therapy, which comprises such modified DNA.
Owner:CSL BEHRING GMBH
Mutant proteins, high potency inhibitory antibodies and fimch crystal structure
InactiveUS20030199071A1Great functional inhibitory activityStrong inhibitory activityHydrolasesImmunoglobulins against bacteriaPassive ImmunizationsMutated protein
The present invention provides bacterial immunogenic agents for administration to humans and non-human animals to stimulate an immune response. It particularly relates to the vaccination of mammalian species, especially human patients, with variants of the E. coli FimCH protein that elicit antibodies that have better functional inhibitory activity than antibodies raised against wild type protein. In particular, such variants include mutations that promote a more open confirmation of the FimH protein, particularly in regions involved in mannose binding, to expose regions previously poorly exposed and mutations that abolish a significantly reduce mannose binding. In another aspect, the invention provides antibodies against such proteins and protein complexes that may be used in passive immunization to protect or treat pathogenic bacterial infections. The present invention also provides machine readable media embedded with the three-dimensional atomic structure coordinates of FimCH bound to mannose, and subsets thereof, and methods of using the crystal structure to provide candidate amino acid residues for mutation.
Owner:WASHINGTON UNIV IN SAINT LOUIS +1
RecA mutants
InactiveUS7176007B2Improve concentrationEliminate requirementsSugar derivativesMicrobiological testing/measurementMutated proteinDouble mutation
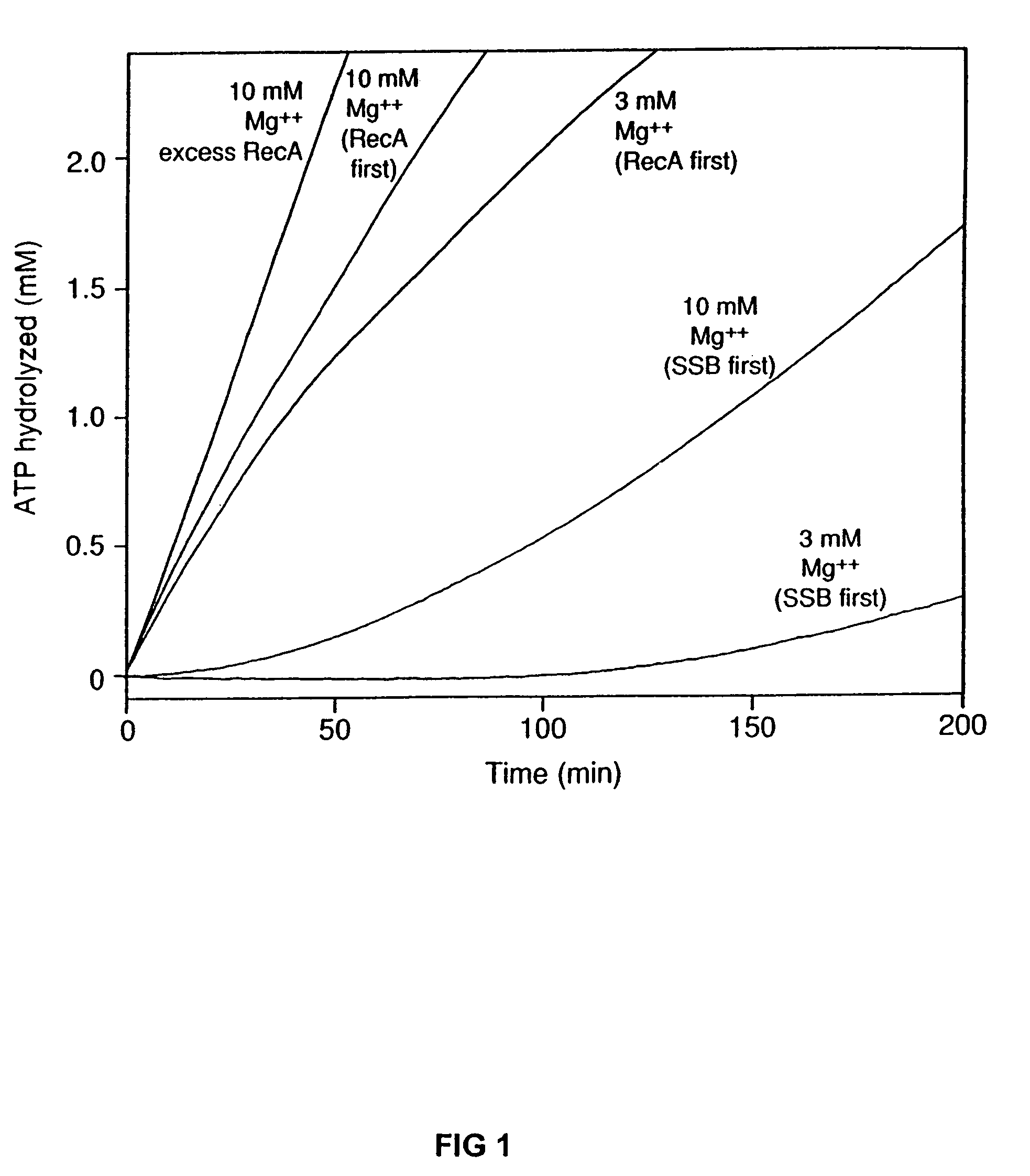
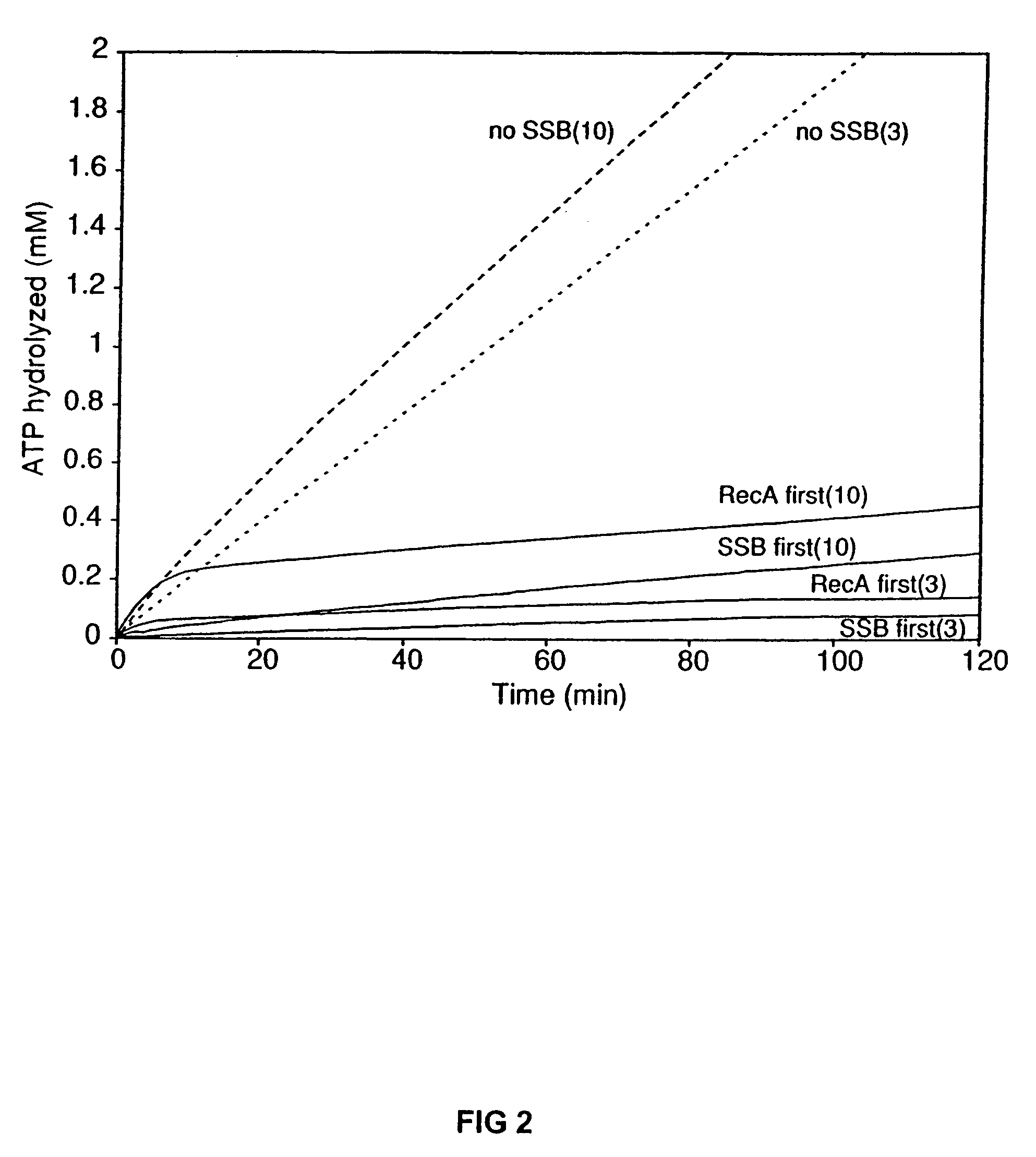
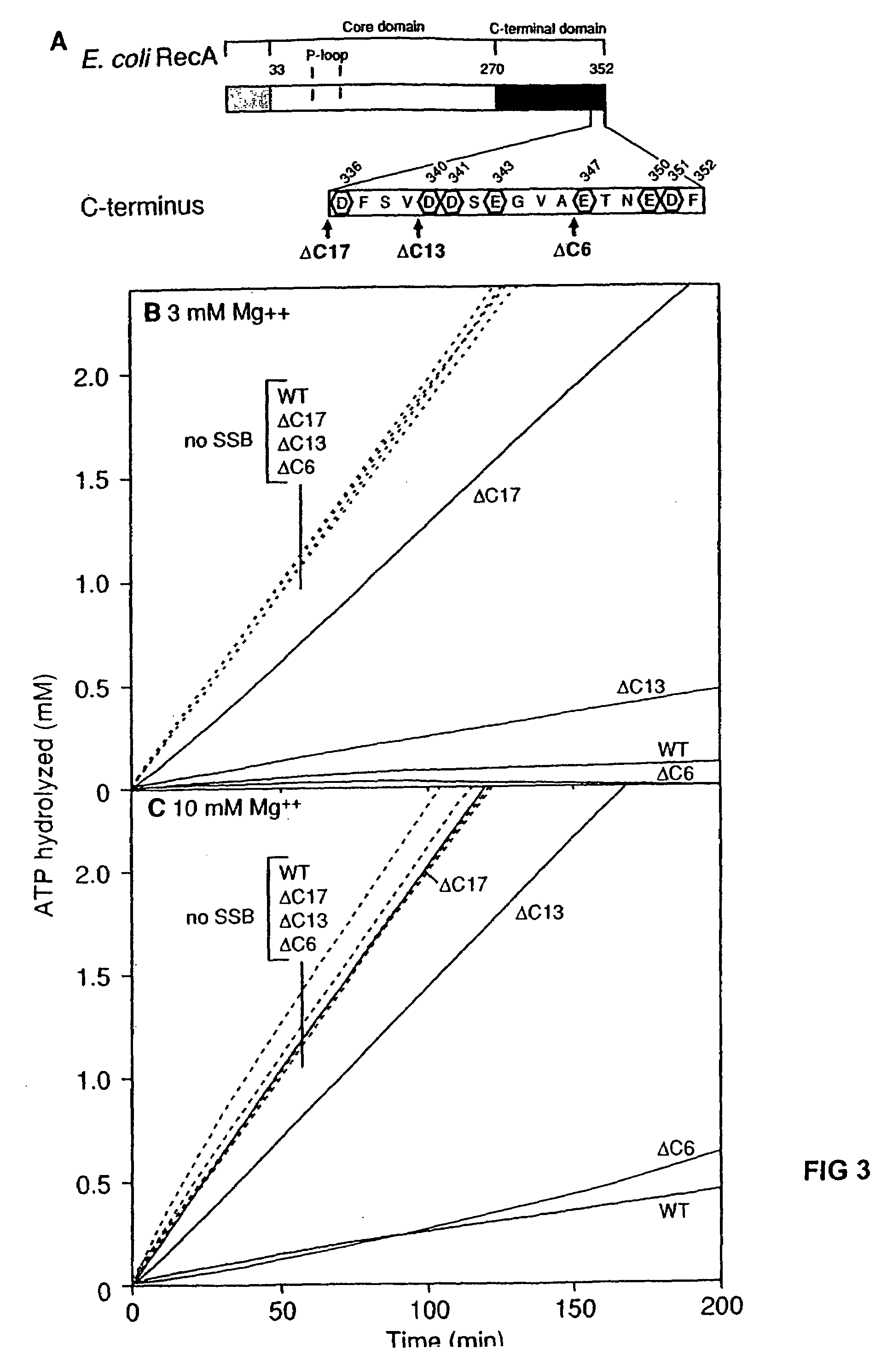
The present invention provides RecA mutant proteins, having either a single mutation or a double mutation. The RecA mutant proteins are highly proficient in both SSB displacement and steady state binding of DNA in the presence or absence of SSB as compared to the wild-type protein. The single RecA mutant, RecAΔC17, has 17 amino acid residues removed from the carboxyl terminus. The double mutant RecA, RecAΔC17 / E38K, combines the 17 amino acid residue C-terminal deletion of RecAΔC17, with a single amino acid change from Glutamate to Lysine at position 38. These RecA mutant proteins are pH sensitive allowing control over formation of products. Hence, methods of using the novel RecA mutants and kits having the RecA mutants as components thereof are also contemplated by the present invention.
Owner:WISCONSIN ALUMNI RES FOUND
Non-transgenic herbicide resistant plants
InactiveUS20030084473A1High sensitivityAlignment score can be increasedTransferasesOther foreign material introduction processesMutated proteinPlant cell
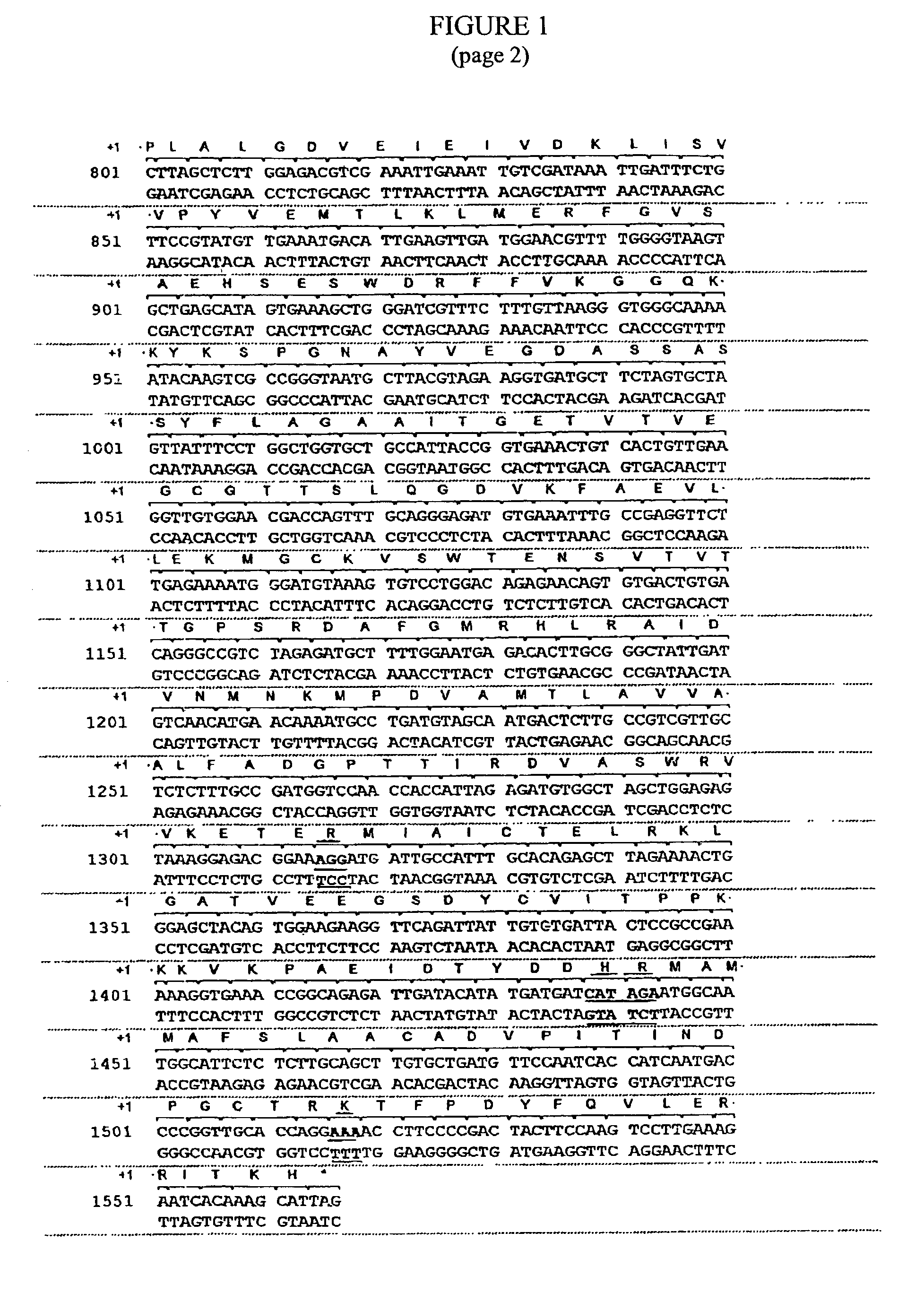
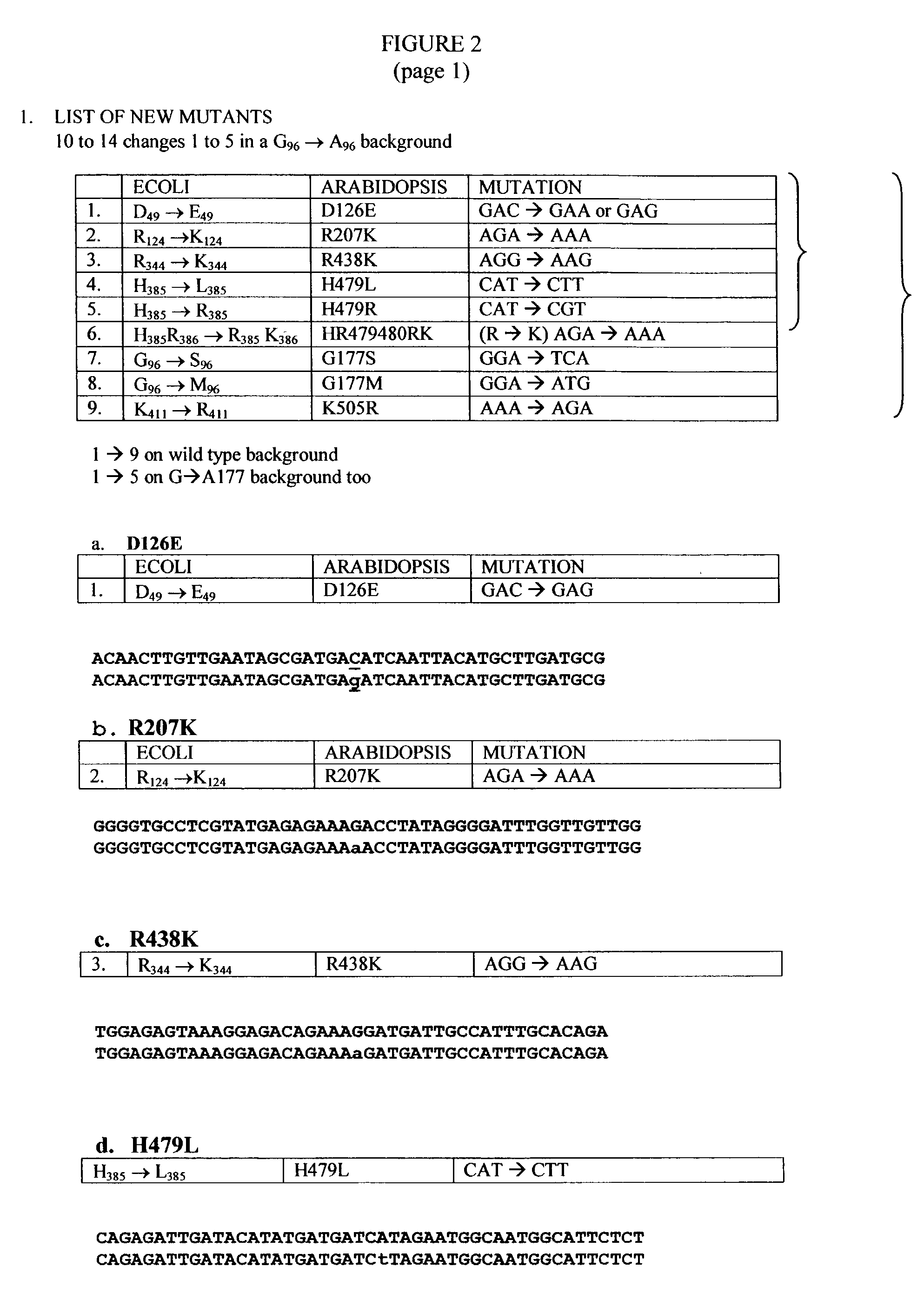
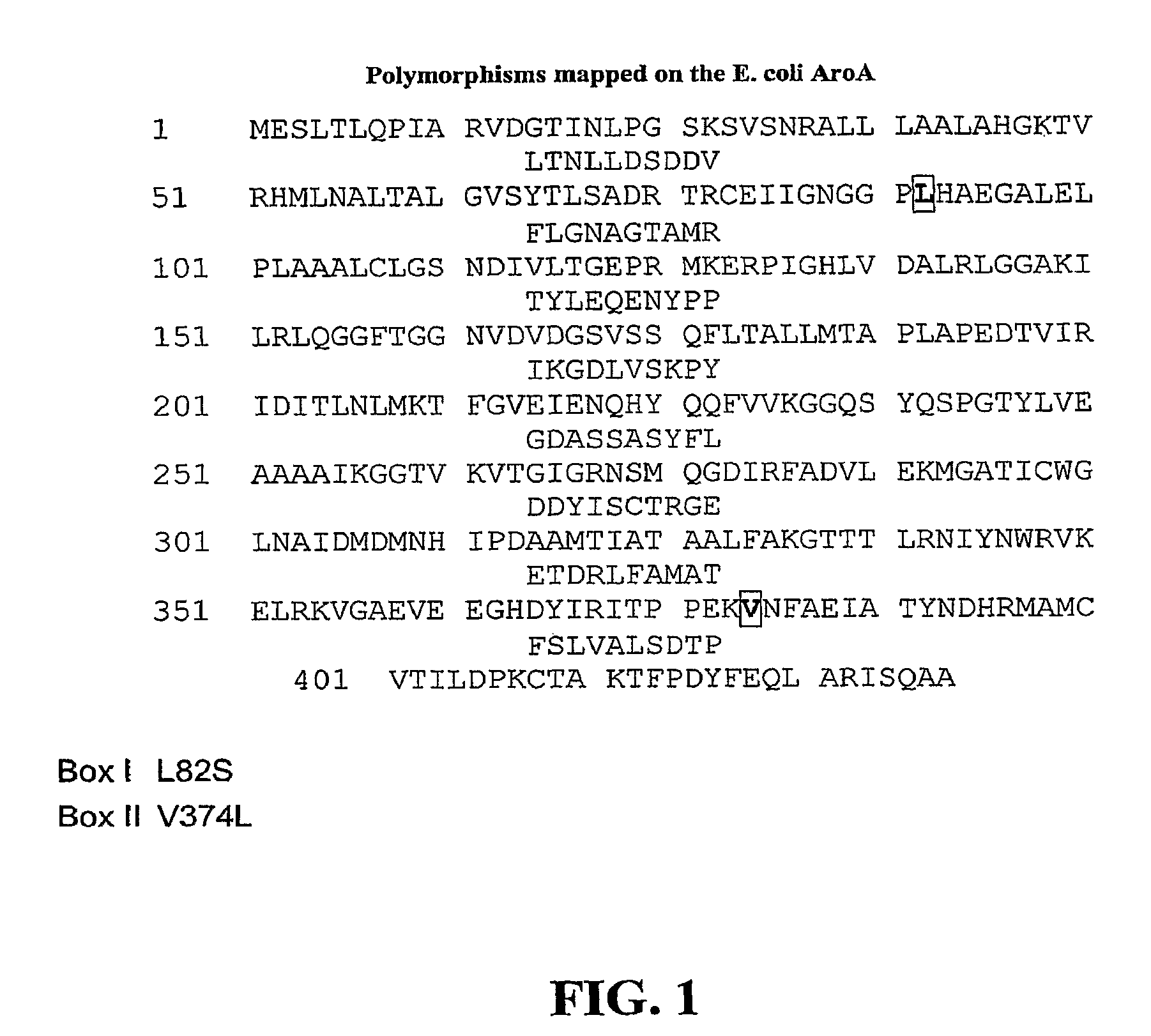
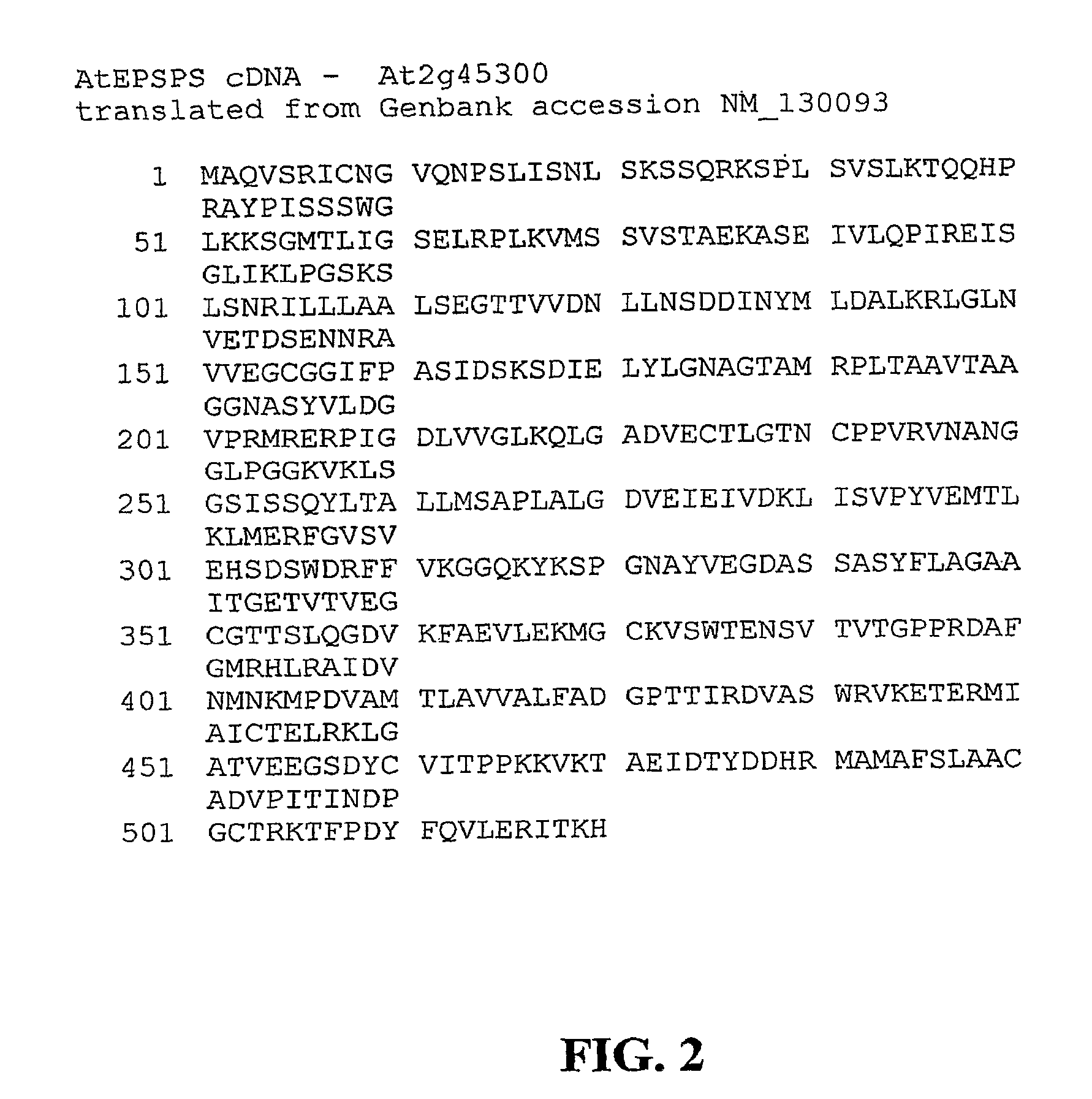
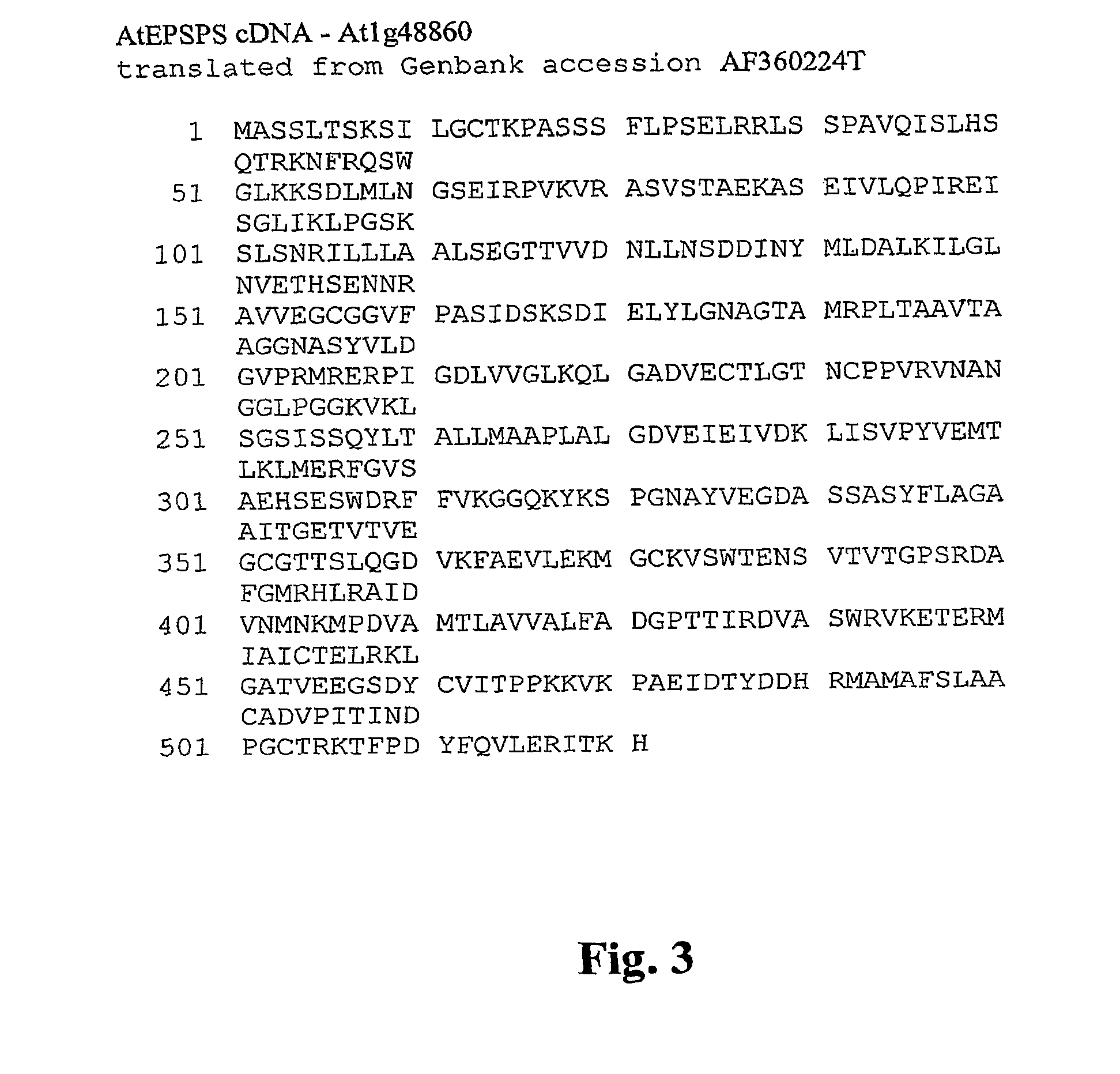
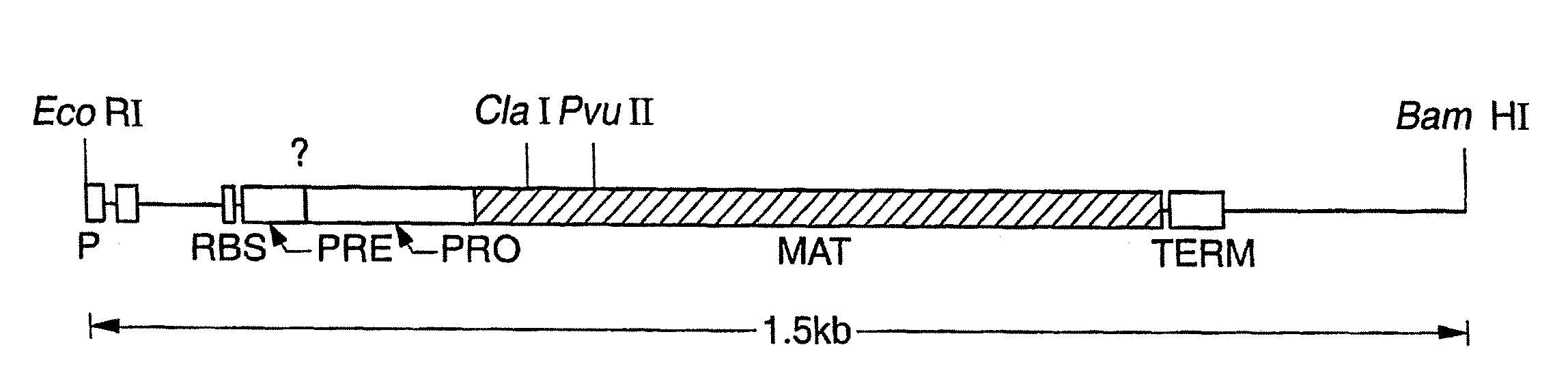
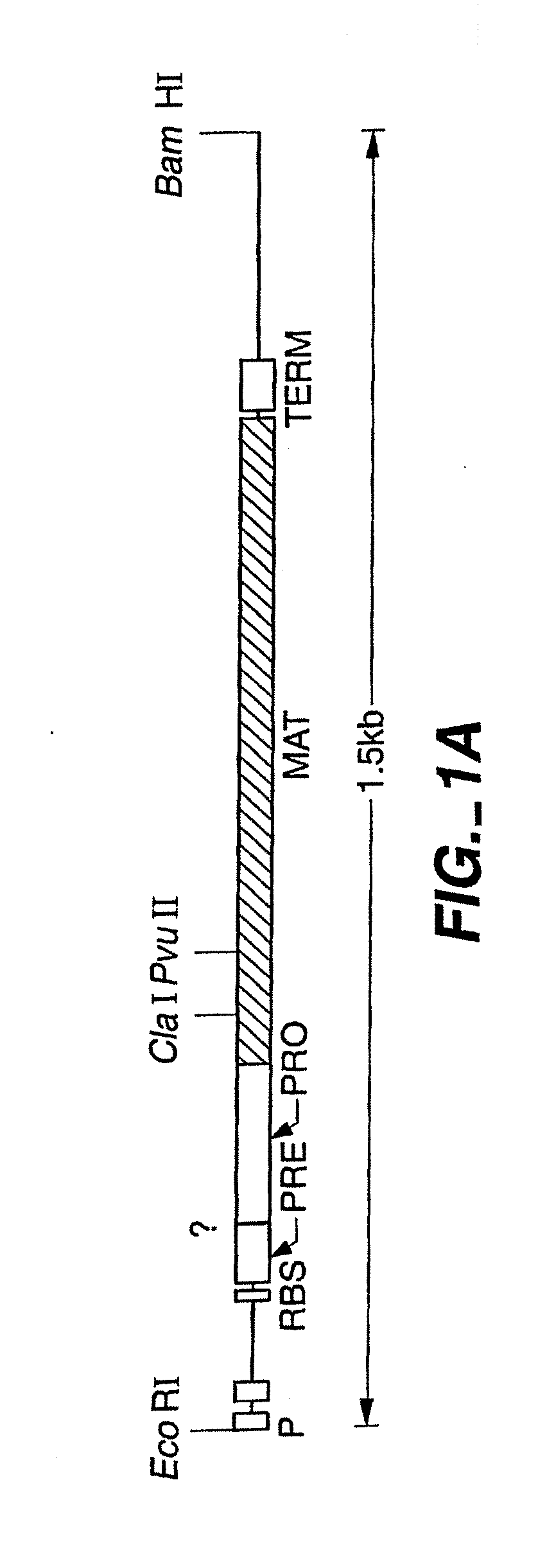
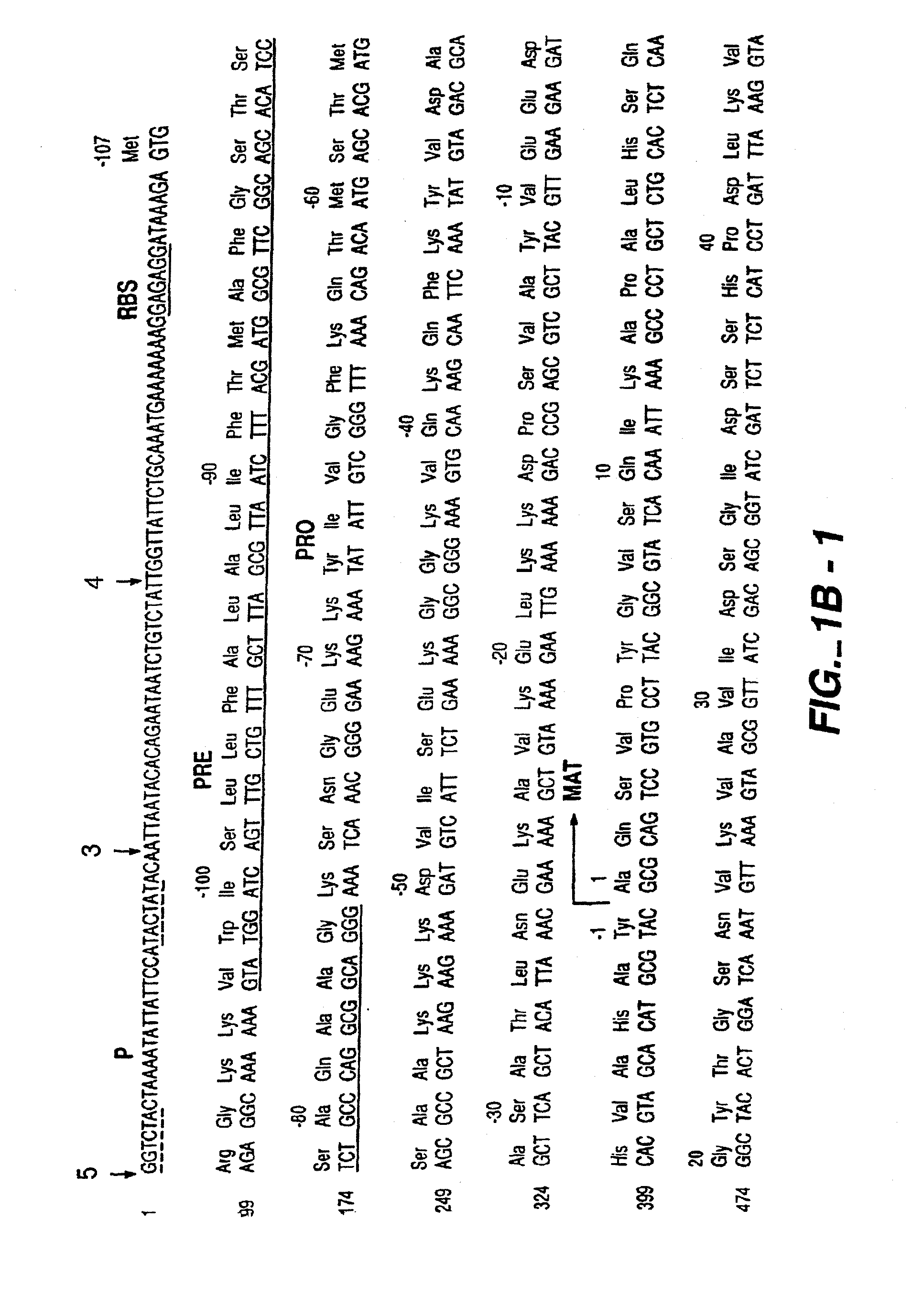
The present invention relates to the production of a non-transgenic plant resistant or tolerant to a herbicide of the phosphonomethylglycine family, e.g., glyphosate. The present invention also relates to the use of a recombinagenic oligonucleobase to make a desired mutation in the chromosomal or episomal sequences of a plant in the gene encoding for 5-enol pyruvylshikimate-3-phosphate synthase (EPSPS). The mutated protein, which substantially maintains the catalytic activity of the wild-type protein, allows for increased resistance or tolerance of the plant to a herbicide of the phosphonomethylglycine family, and allows for the substantially normal growth or development of the plant, its organs, tissues or cells as compared to the wild-type plant irrespective of the presence or absence of the herbicide. The present invention also relates to a non-transgenic plant cell in which the EPSPS gene has been mutated, a non-transgenic plant regenerated therefrom, as well as a plant resulting from a cross using a regenerated non-transgenic plant having a mutated EPSPS gene. The amino acids at the positions 126, 177, 207, 438, 479, 480 and / or 505 are changed tp produce a mutant EPSPS gene product.
Owner:VALIGEN US
Glycosylated modified primate lentivirus envelope polypeptides
InactiveUS6908617B1Improving immunogenicityPeptide/protein ingredientsViral antigen ingredientsAdjuvantBinding site
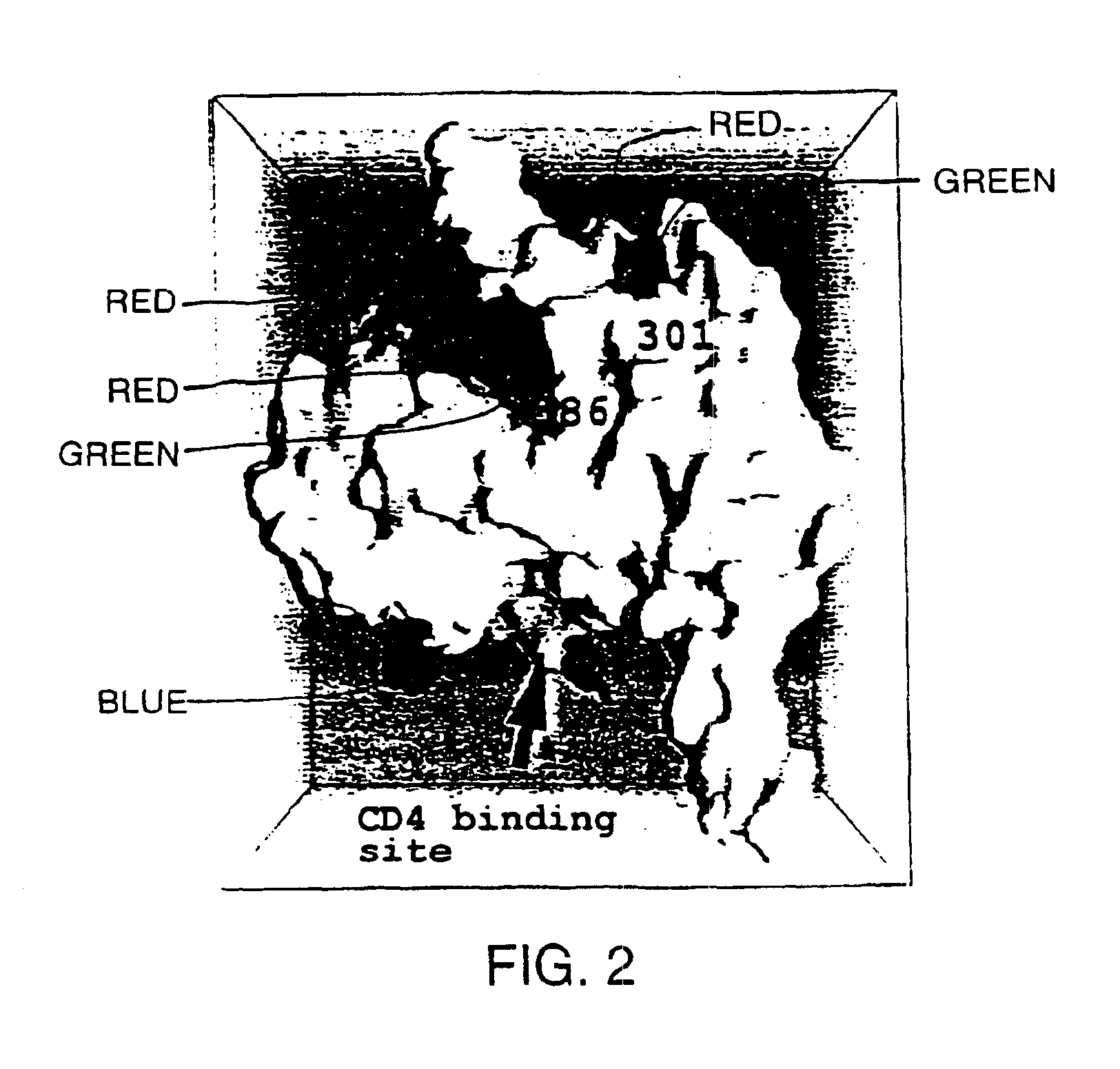
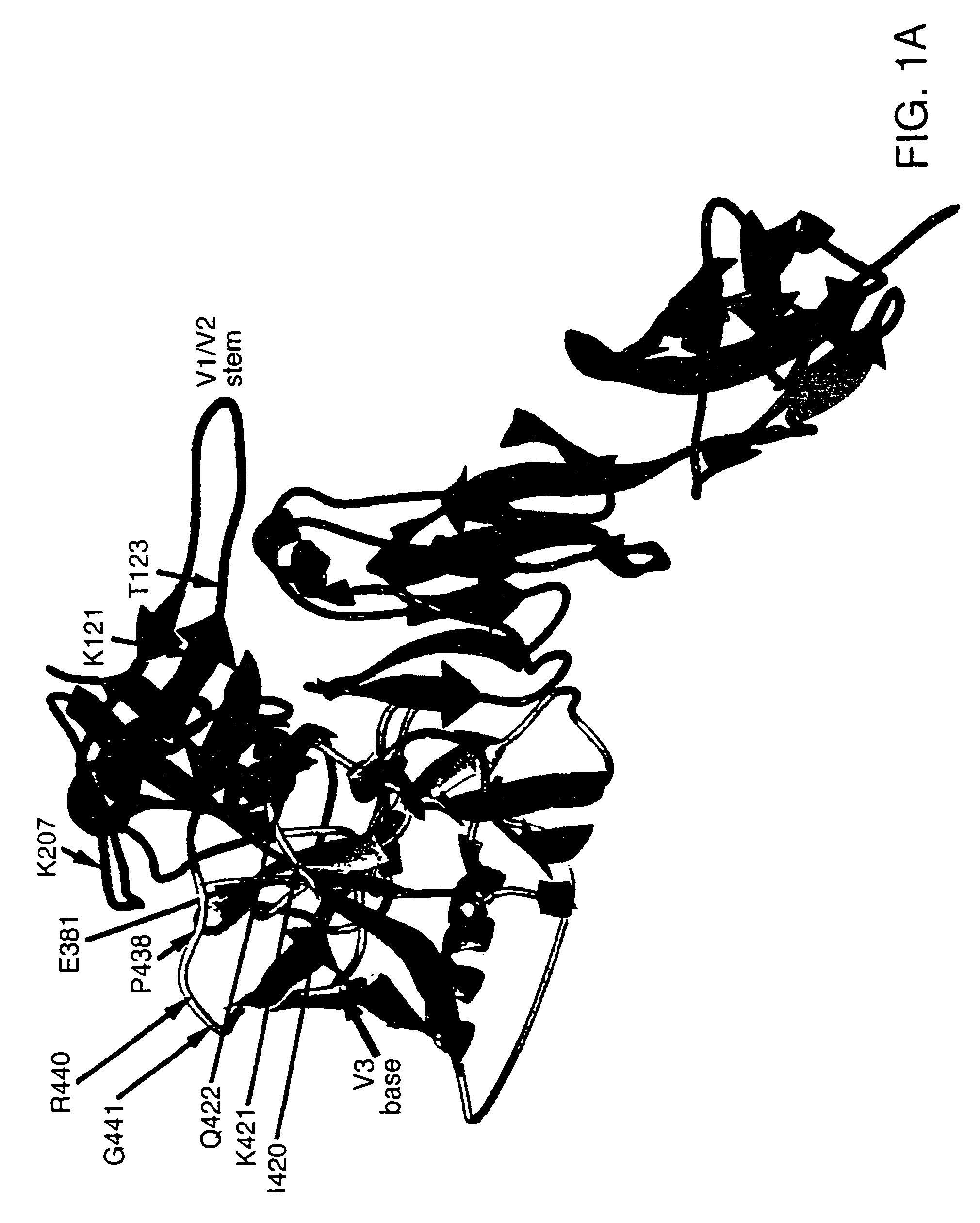
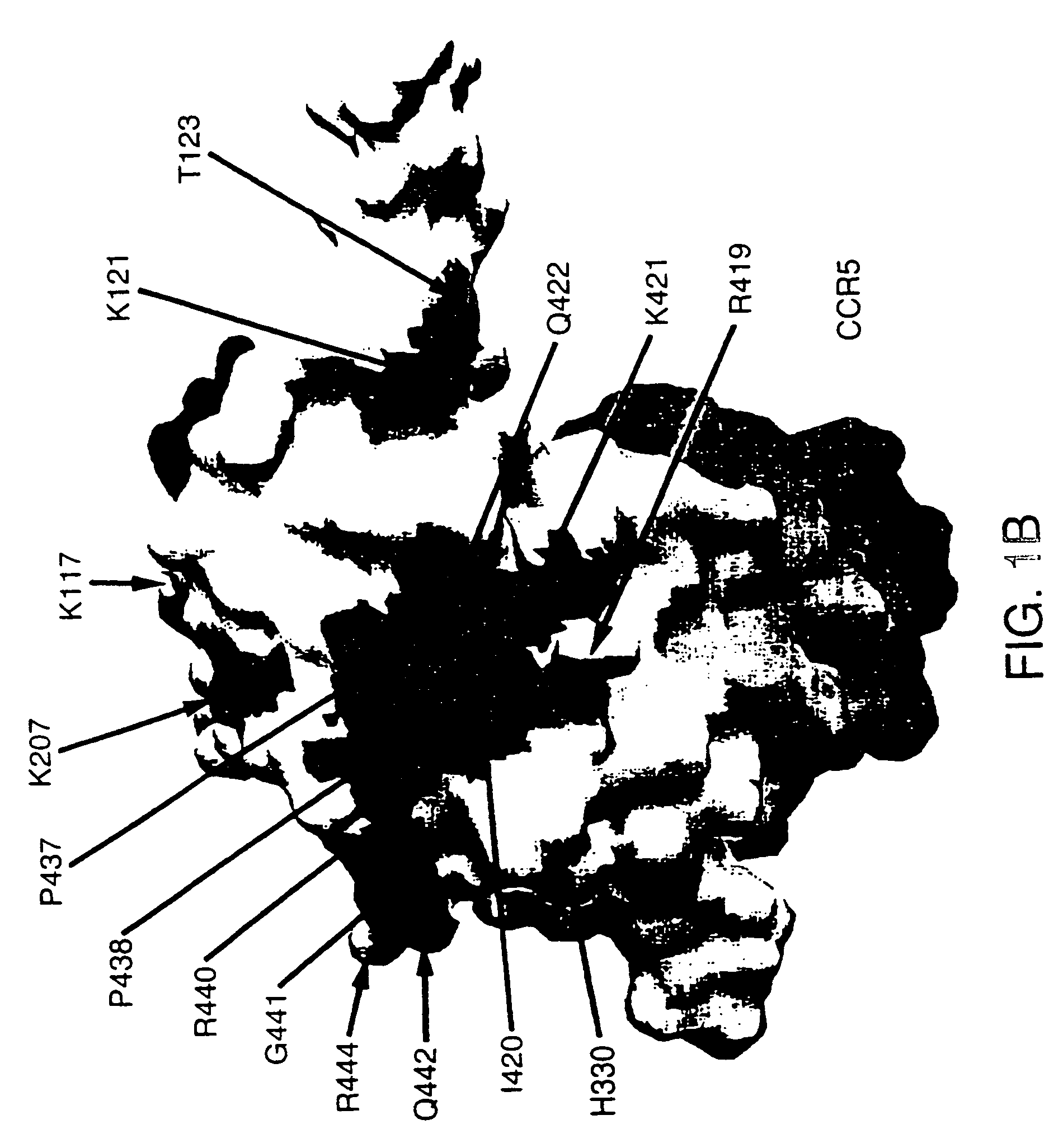
A modified polypeptide corresponding to an envelope glycoprotein of a primate lentivirus is described. The polypeptide has been modified from the wild-type structure so that it has at least two of the glycosylation sites proximal to the CD4 binding site or chemokine receptor site altered so that the alteration prevents glycosylation at that site or where glycosylation sites distal to these sites have been derivatized with a molecular adjuvant, while retaining the overall 3-dimensional structure of a discontinuous conserved epitope of the wild-type protein. Preferably, the polypeptide has both changes. Preferably, the primate lentivirus is HIV, and the protein is HIV-1 gp 120.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK +1
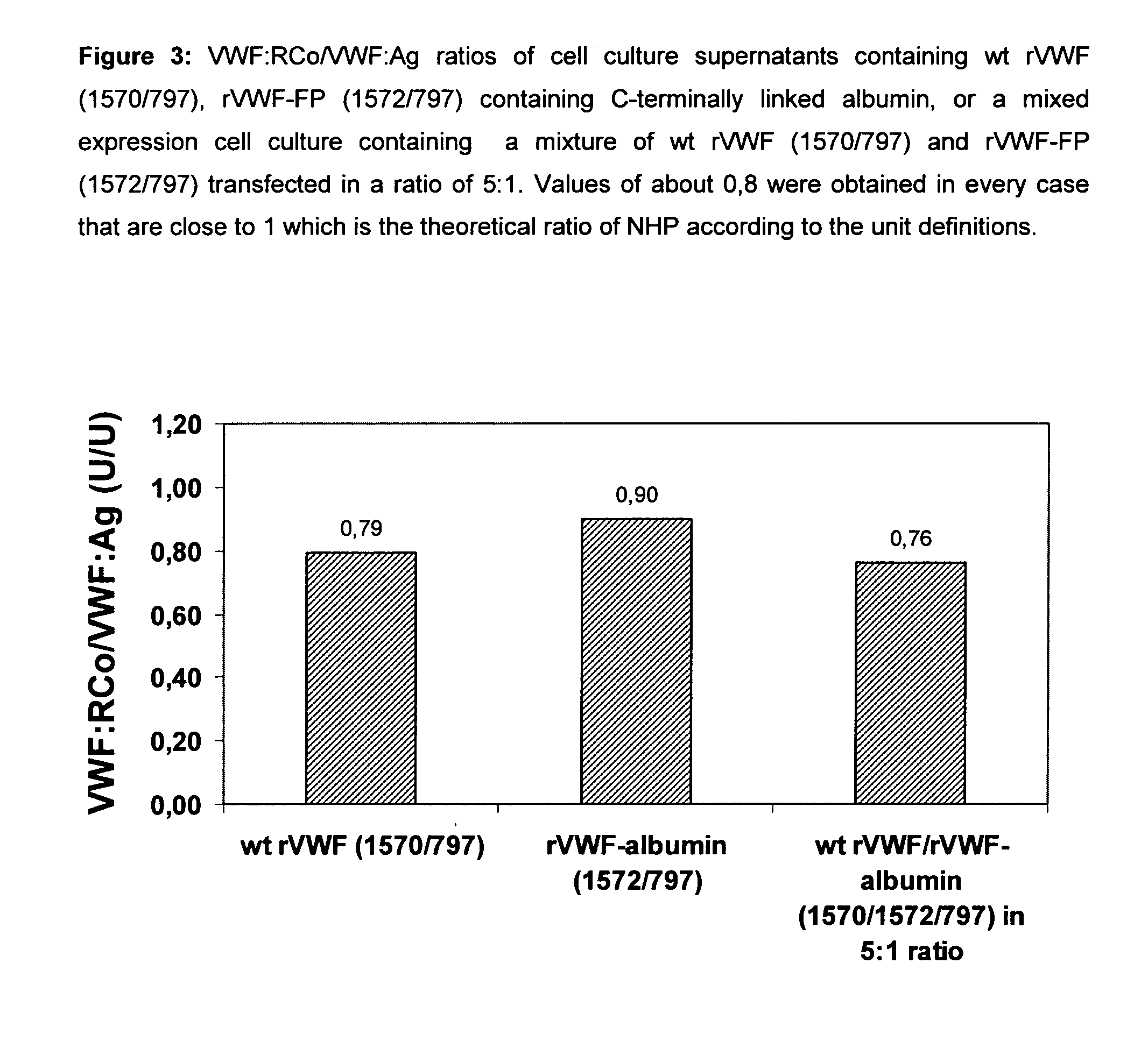
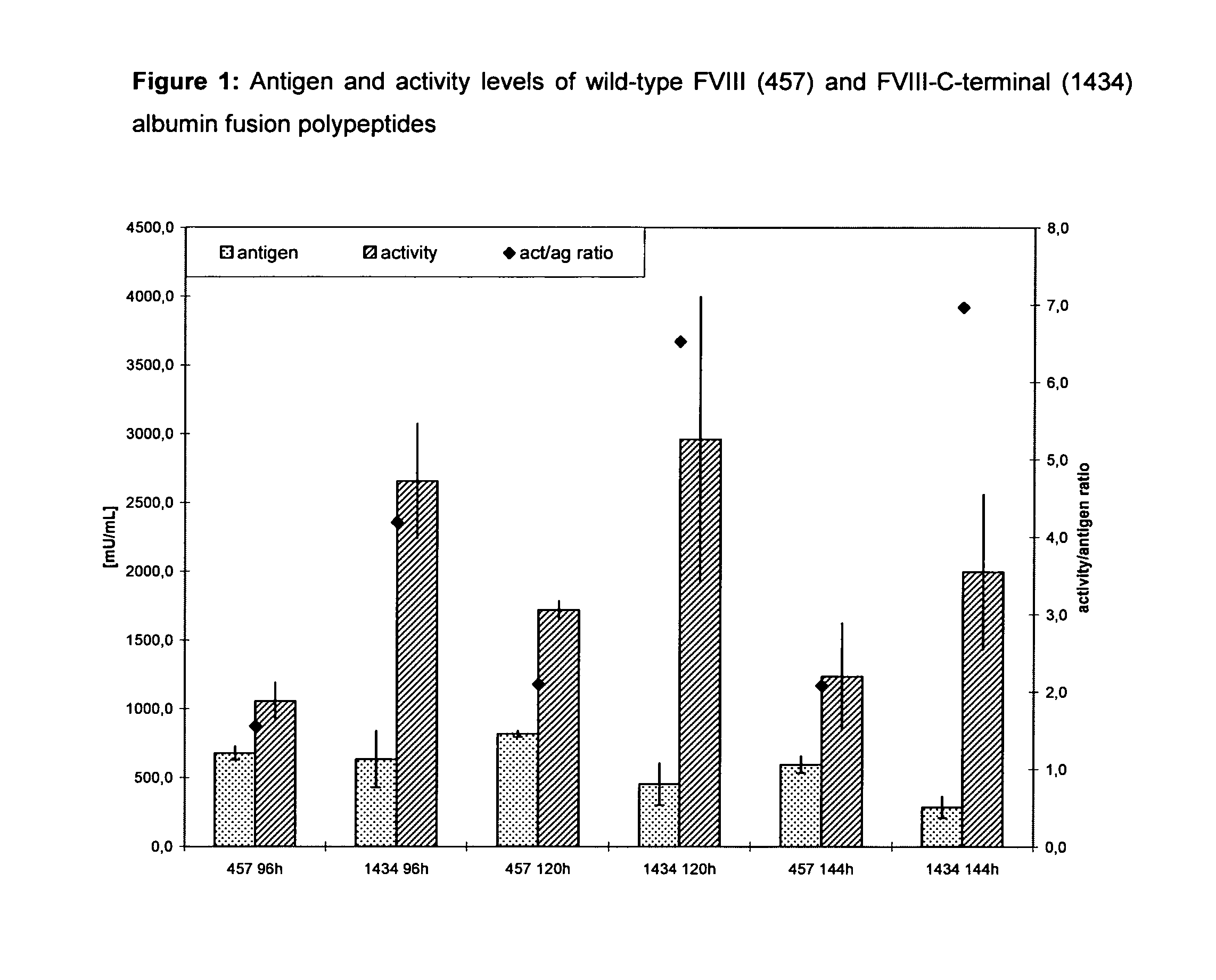
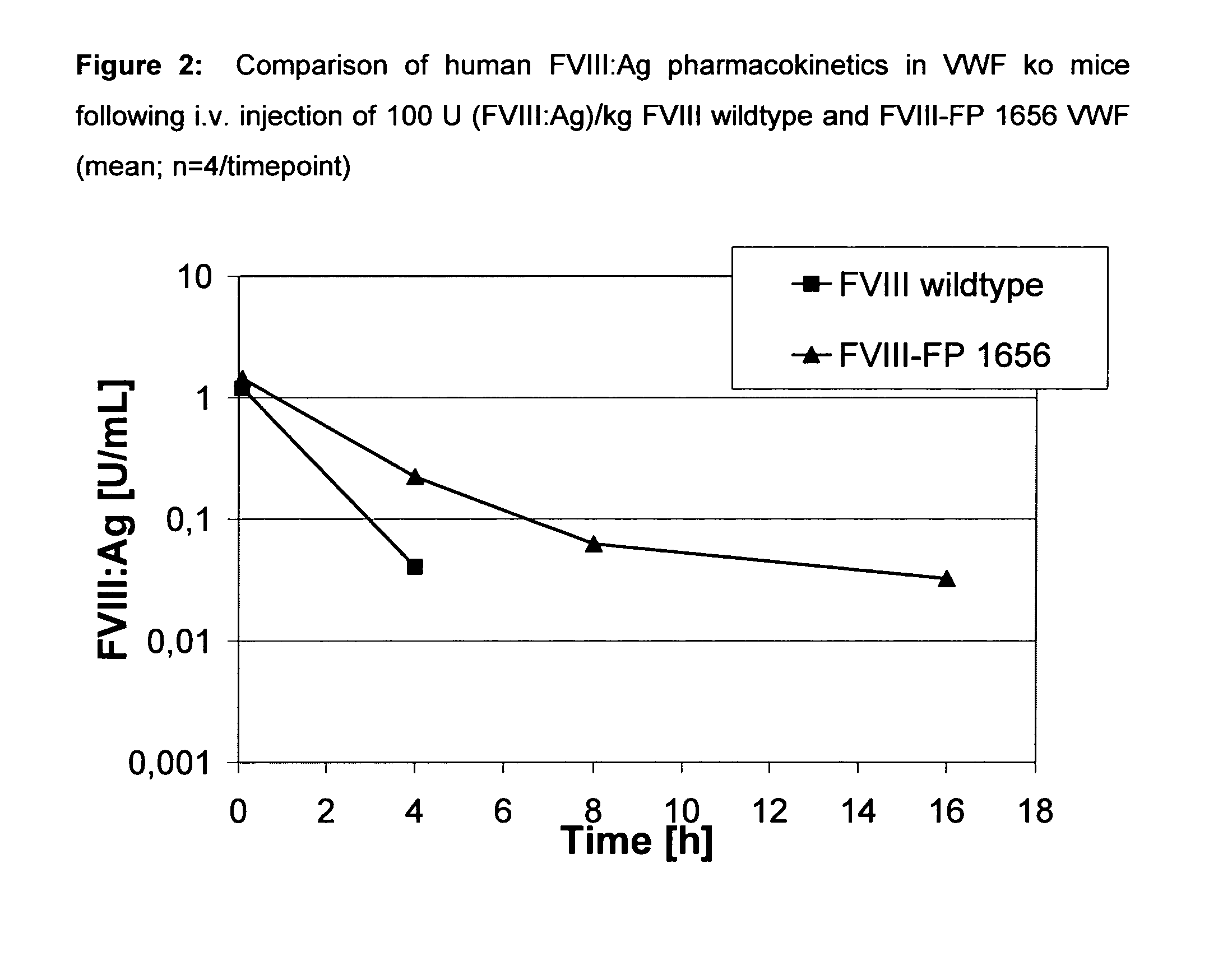
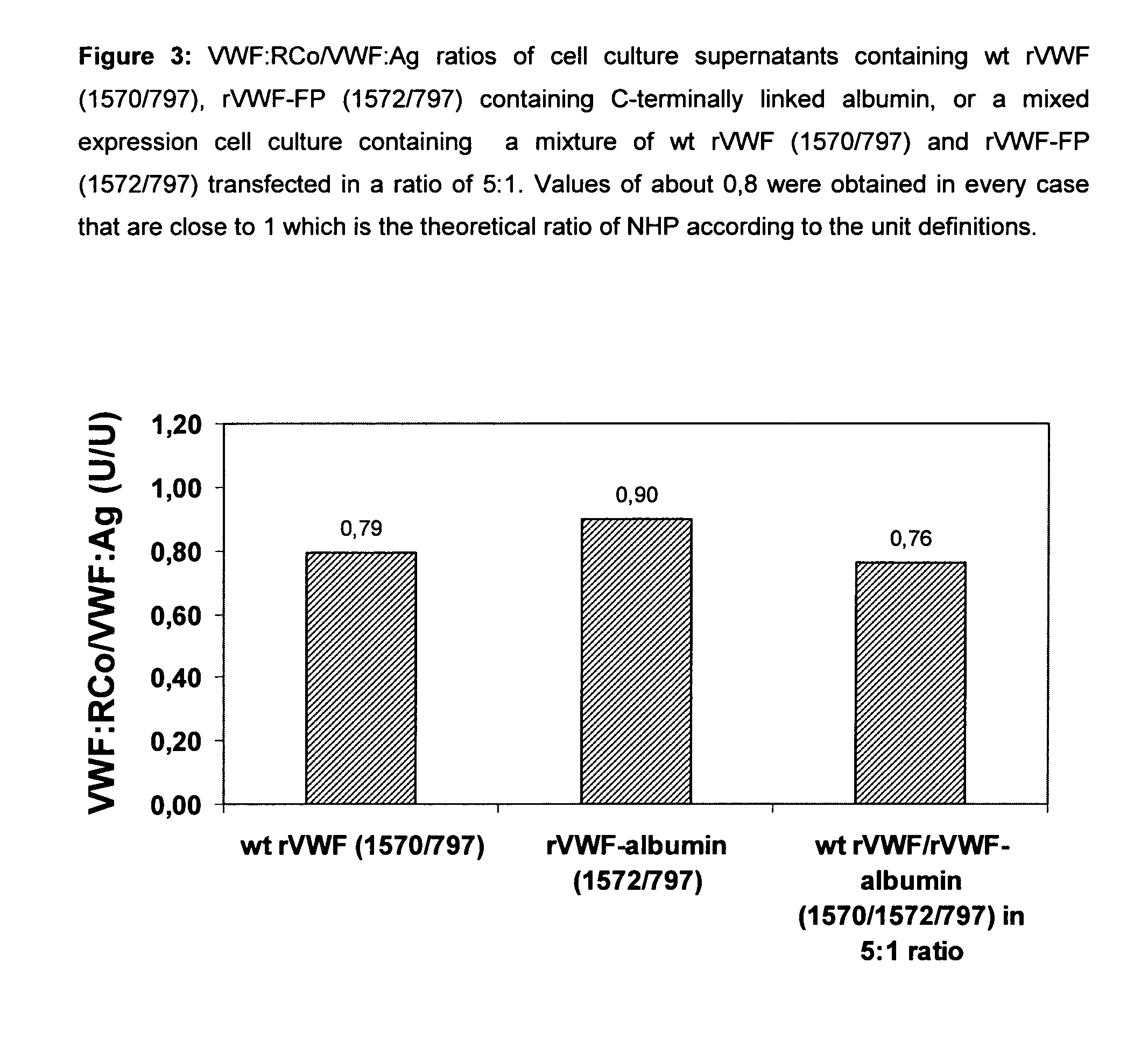
Factor viii, von willebrand factor or complexes thereof with prolonged in vivo half-life
ActiveUS20110183907A1Retain biological activityPromote recoveryOrganic active ingredientsFactor VIIFactor VIII vWFNucleic acid sequencing
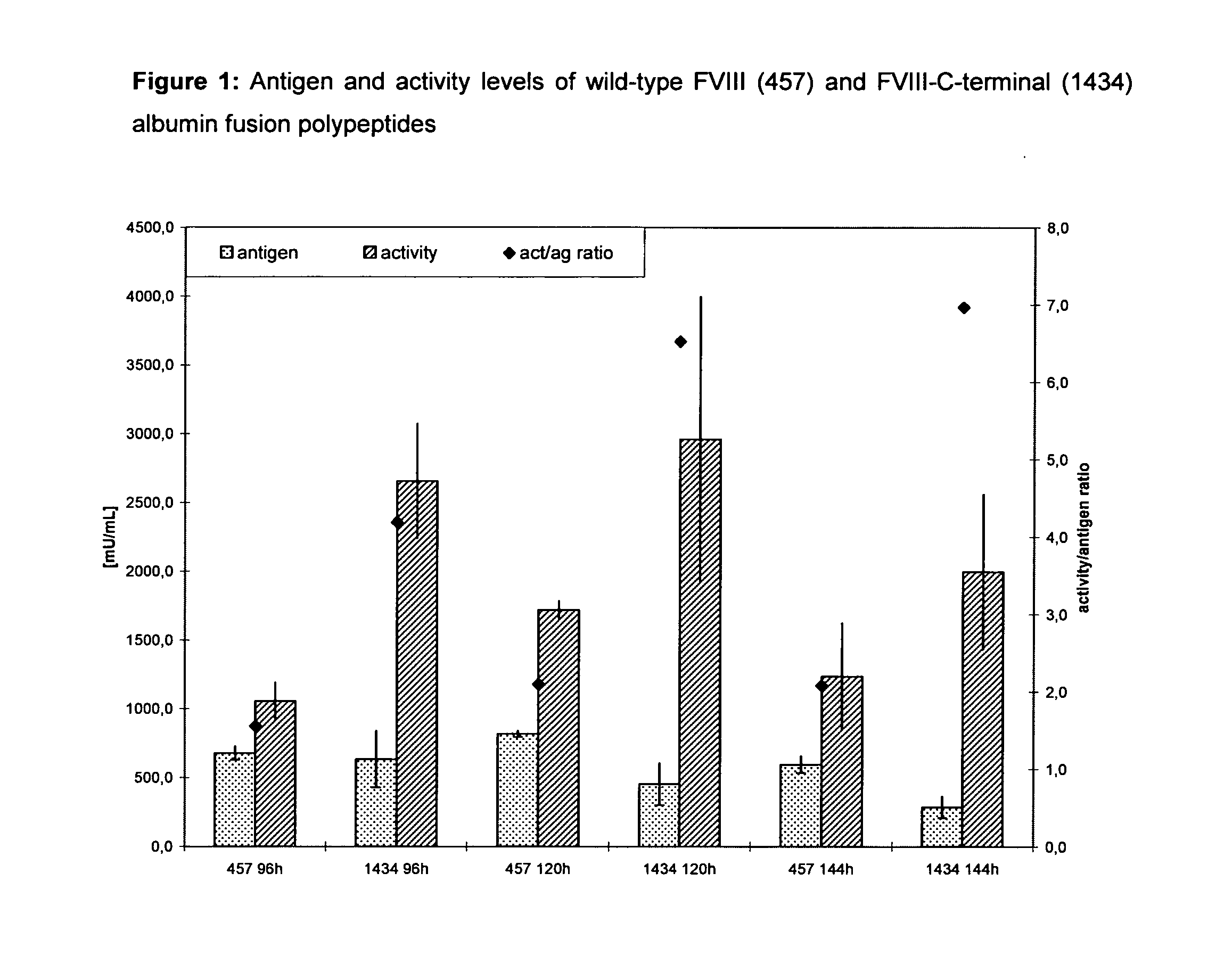
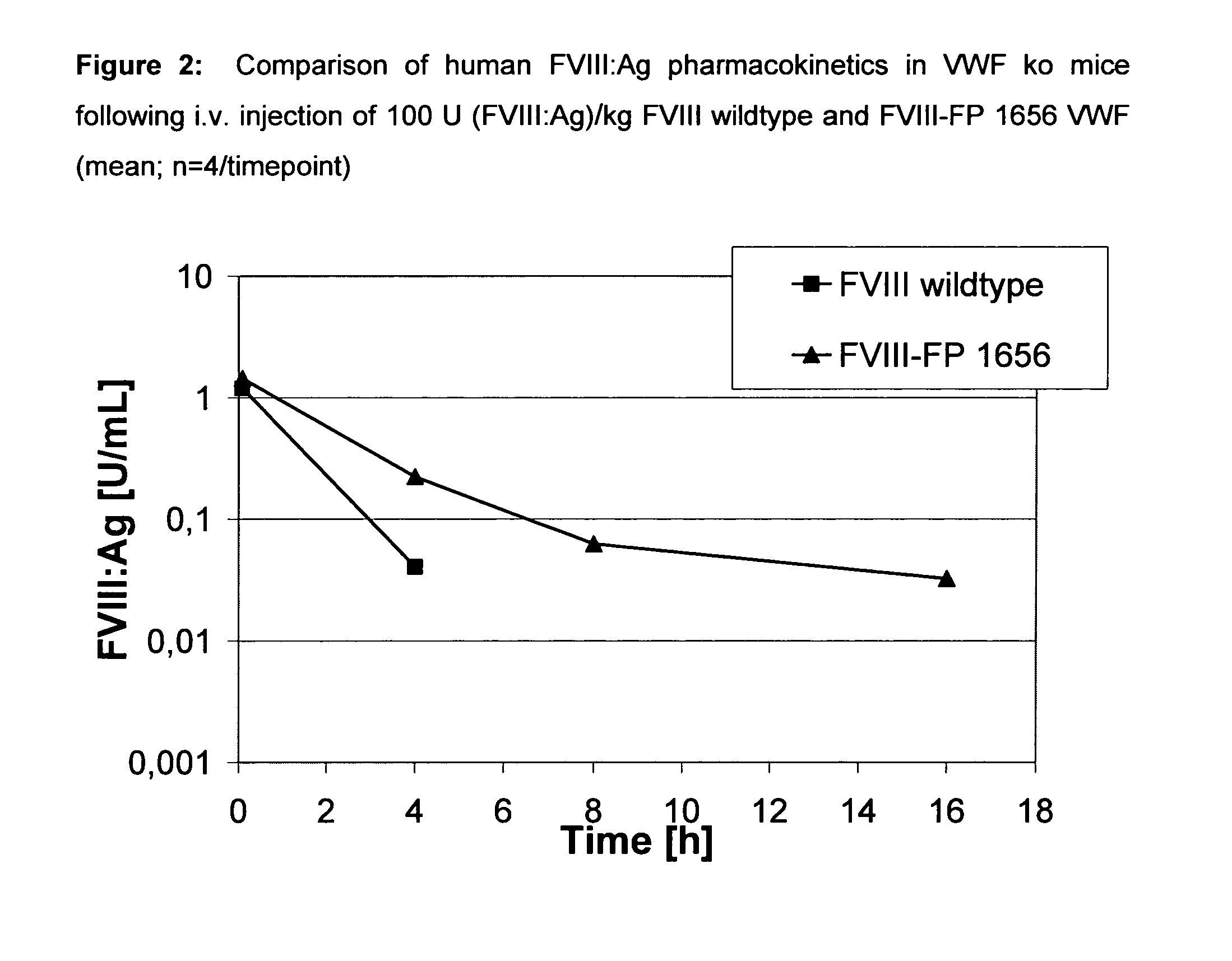
The present invention relates to modified nucleic acid sequences coding for coagulation factor VIII (FVIII) and for von Willebrand factor (VWF) as well as complexes thereof and their derivatives, recombinant expression vectors containing such nucleic acid sequences, host cells transformed with such recombinant expression vectors, recombinant polypeptides and derivatives coded for by said nucleic acid sequences which recombinant polypeptides and derivatives do have biological activities together with prolonged in vivo half-life and / or improved in vivo recovery compared to the unmodified wild-type protein. The invention also relates to corresponding FVIII sequences that result in improved expression yield. The present invention further relates to processes for the manufacture of such recombinant proteins and their derivatives. The invention also relates to a transfer vector for use in human gene therapy, which comprises such modified nucleic acid sequences.
Owner:CSL BEHRING GMBH
Protease producing an altered immunogenic response and methods of making and using the same
InactiveUS20050148059A1Easy to identifyRaise security concernsCosmetic preparationsFungiProteinase activityDna encoding
Owner:GENENCOR INT INC
Mirac Proteins
This disclosure relates to a method of generating conditionally active biologic proteins from wild type proteins, in particular therapeutic proteins, which are reversibly or irreversibly inactivated at the wild type normal physiological conditions. For example, evolved proteins are virtually inactive at body temperature, but are active at lower temperatures.
Owner:BIOATLA LLC
Stabilized primate lentivirus envelope glycoproteins
InactiveUS7048929B1Improve stabilityImproving immunogenicityAntibody mimetics/scaffoldsVirus peptidesEnv GlycoproteinsDisulfide bond
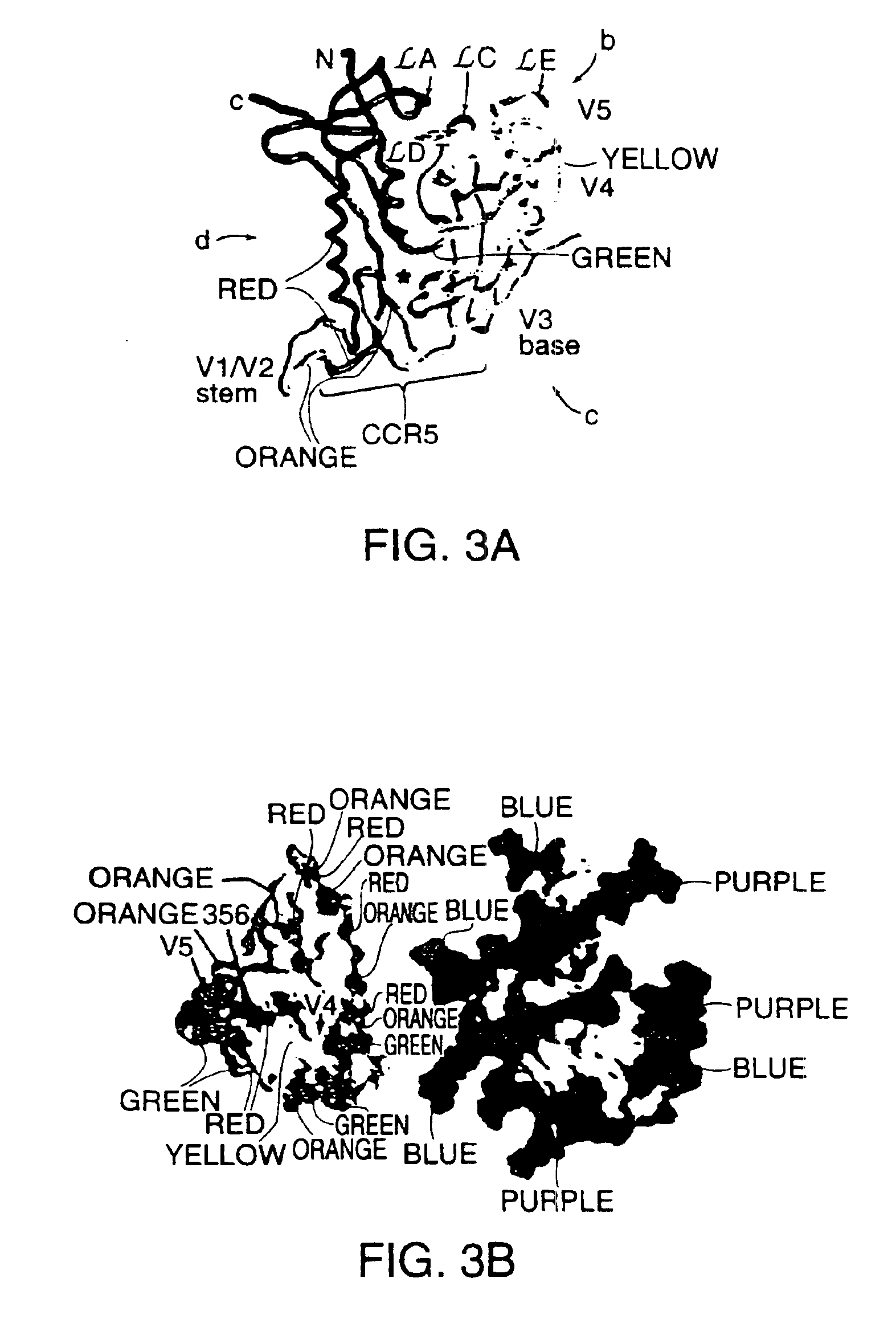
A modified polypeptide corresponding to an envelope glycoprotein of a primate lentivirus is described. The polypeptide has been modified from the wild-type structure so that it has cysteine amino acid residues introduced to create disulfide bonds, a cavity is filled with hydrophobic amino acids, a Proresidue is introduced at a defined turn structure of the protein, or the hydrophobicity is increased across the interface between different domains, while retaining the overall 3-dimensional structure of a discontinuous conserved epitope of the wild-type protein. Preferably, the polypeptide has more than one of those characteristics. Preferably, the primate lentivirus is HIV, and the protein is HIV-1 gp120.
Owner:DANA FARBER CANCER INST INC +1
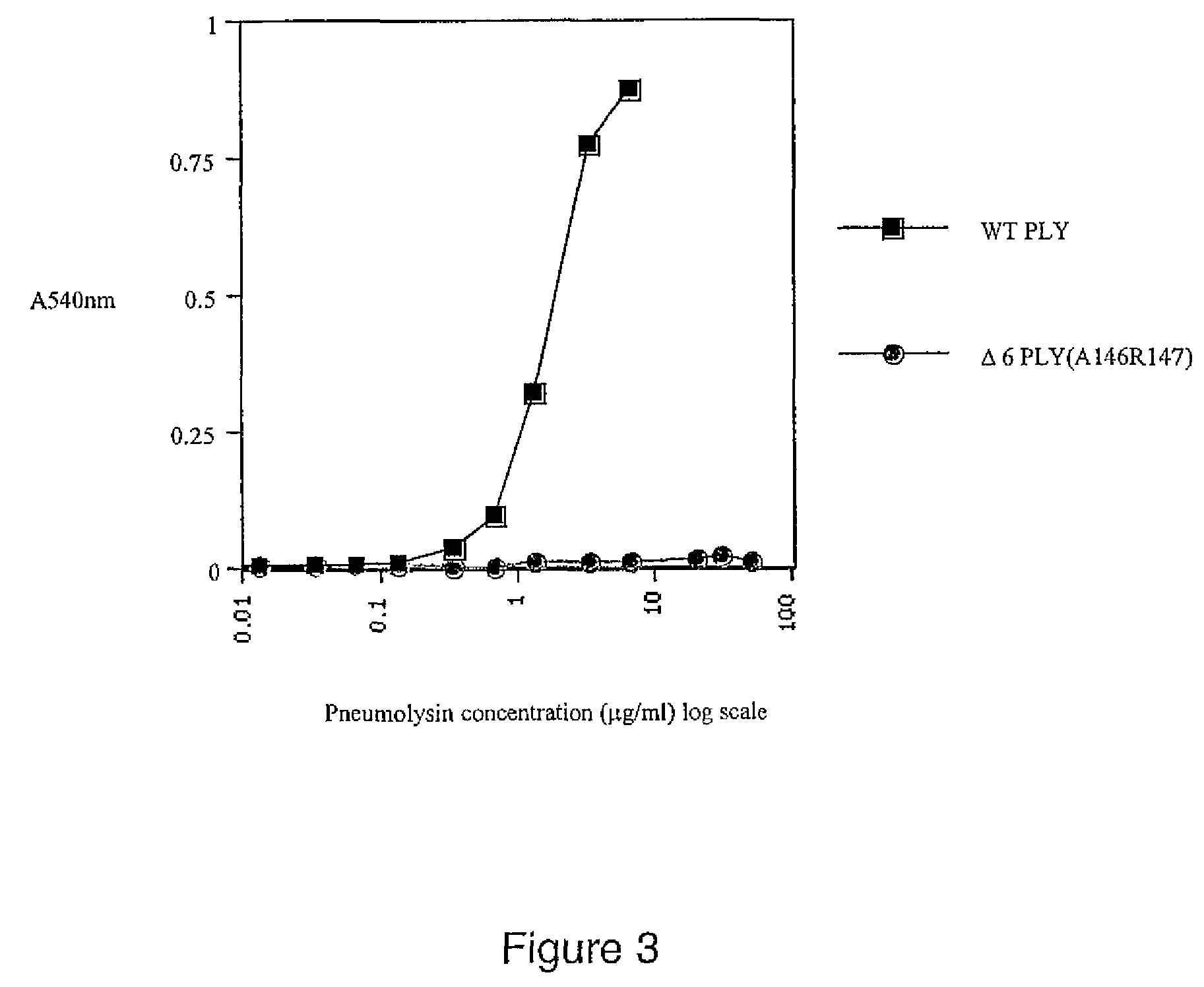
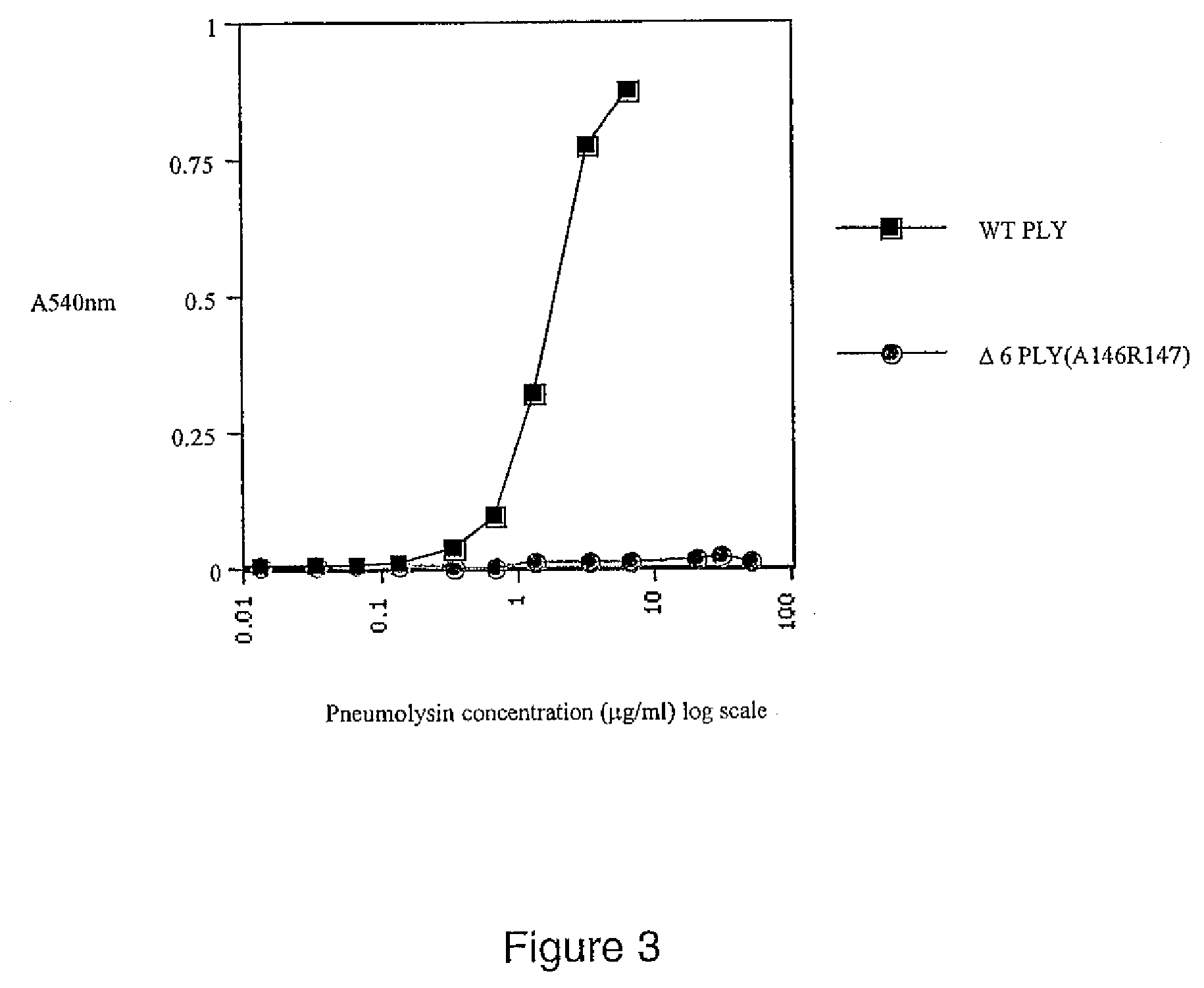
Mutant pneumolysin proteins
InactiveUS7820789B2Low toxicityReduced oligomerisation activityAntibacterial agentsPeptide/protein ingredientsSynechococcusPyrococcus
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW
Factor VIII, von willebrand factor or complexes thereof with prolonged in vivo half-life
ActiveUS8575104B2Prolong half-life in vivoFactor VIIPeptide/protein ingredientsFactor VIII vWFHalf-life
The present invention relates to modified nucleic acid sequences coding for coagulation factor VIII (FVIII) and for von Willebrand factor (VWF) as well as complexes thereof and their derivatives, recombinant expression vectors containing such nucleic acid sequences, host cells transformed with such recombinant expression vectors, recombinant polypeptides and derivatives coded for by said nucleic acid sequences which recombinant polypeptides and derivatives do have biological activities together with prolonged in vivo half-life and / or improved in vivo recovery compared to the unmodified wild-type protein. The invention also relates to corresponding FVIII sequences that result in improved expression yield. The present invention further relates to processes for the manufacture of such recombinant proteins and their derivatives. The invention also relates to a transfer vector for use in human gene therapy, which comprises such modified nucleic acid sequences.
Owner:CSL BEHRING GMBH
Proteases producing an altered immunological response and methods of making and using the same
The present invention provides novel protein variants that exhibit reduced immunogenic responses, as compared to the parental proteins. The present invention further provides DNA molecules that encode novel variants, host cells comprising DNA encoding novel variants, as well as methods for making proteins less allergenic. In addition, the present invention provides various compositions that comprise these proteins that are less immunogenic than the wild-type proteins.
Owner:GENENCOR INT INC
EPSPS mutants
The present invention relates to the production of a non-transgenic plant resistant or tolerant to a herbicide of the phosphonomethylglycine family, e.g., glyphosate. The present invention also relates to the use of a recombinagenic oligonucleobase to make a desired mutation in the chromosomal or episomal sequences of a plant in the gene encoding for 5-enol pyruvylshikimate-3-phosphate synthase (EPSPS). The mutated protein, which substantially maintains the catalytic activity of the wild-type protein, allows for increased resistance or tolerance of the plant to a herbicide of the phosphonomethylglycine family, and allows for the substantially normal growth or development of the plant, its organs, tissues or cells as compared to the wild-type plant irrespective of the presence or absence of the herbicide. Additionally the present invention relates to mutant E. coli cells that contain mutated EPSPS genes.
Owner:CIBUS
Proteases producing an altered immunogenic response and methods of making and using the same
InactiveUS20090060933A1Reduced immunogenic responseLess allergicBacterial antigen ingredientsBacteriaProteinase activityDna encoding
The present invention provides novel protein variants that exhibit reduced immunogenic responses, as compared to the parental proteins. The present invention further provides DNA molecules that encode novel variants, host cells comprising DNA encoding novel variants, as well as methods for making proteins less allergenic. In addition, the present invention provides various compositions that comprise these proteins that are less immunogenic than the wild-type proteins.
Owner:ESTELL DAVID A +1
Polynucleotides and polypeptides of the IFNalpha-14 gene
The present invention relates to new polynucleotides derived from the nucleotide sequence of the IFNalpha-14 gene comprising new SNPs, and new polypeptides derived from the natural wild-type IFNalpha-14 protein comprising at least one mutation caused by at least one SNP of the invention as well as their therapeutic uses.
Owner:GENODYSSEE SA
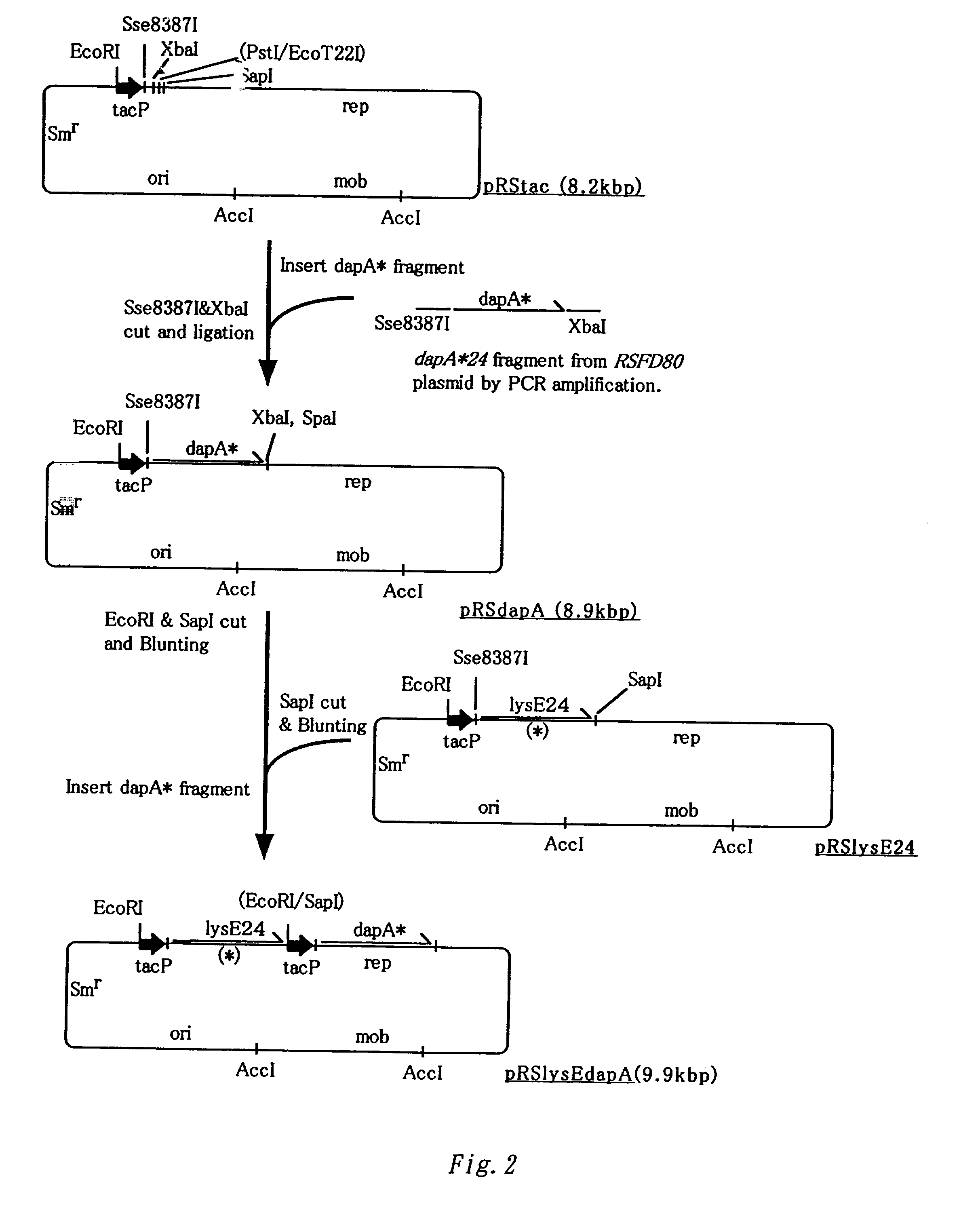
Method for producing L-lysine or L-arginine by using methanol assimilating bacterium
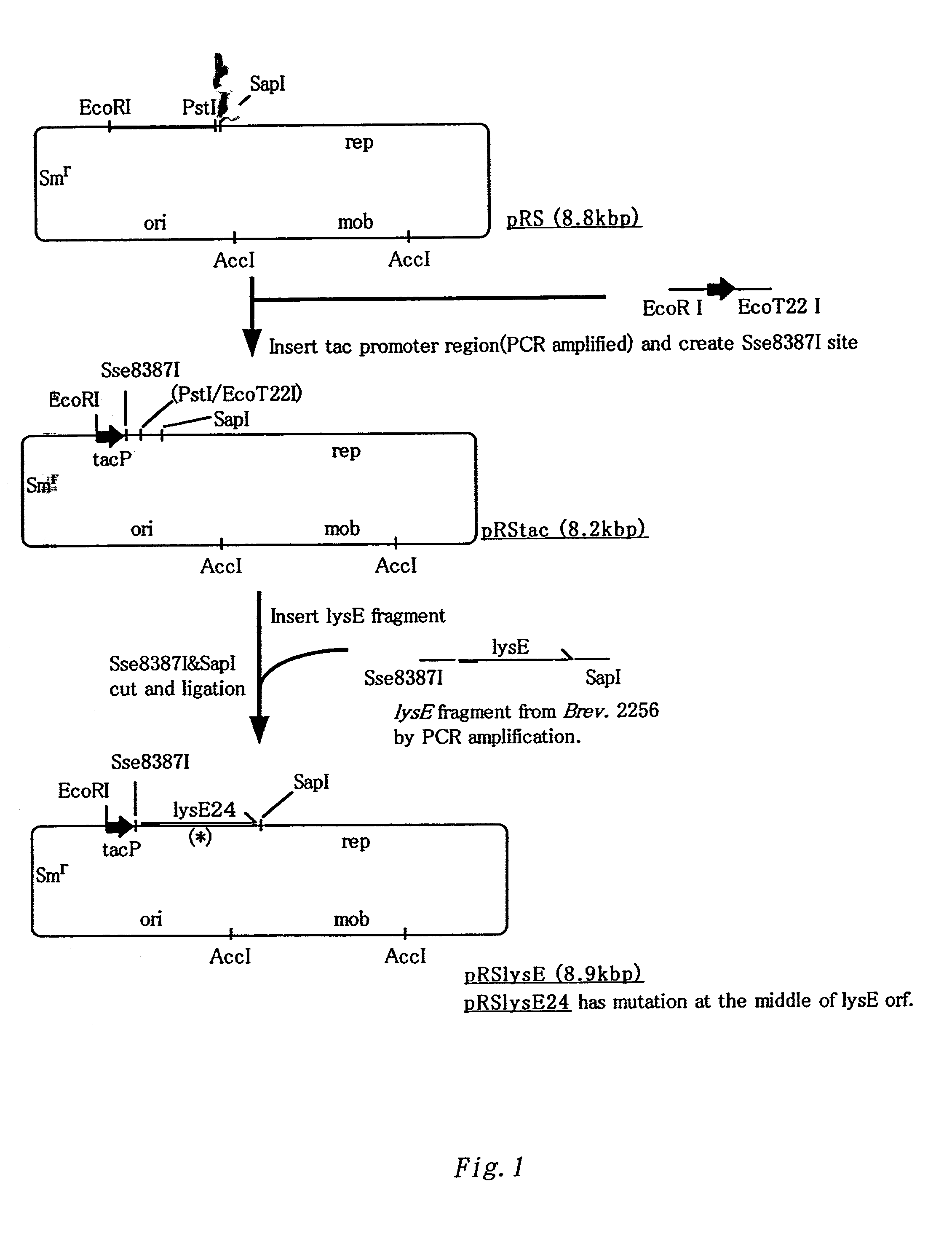
A DNA encoding a variant of a protein, having a loop region and six hydrophobic helixes and involved in excretion of L-lysine to outside of a cell, wherein the DNA encodes a mutant protein not containing the loop region that is contained in a wild-type protein and facilitates excretion of L-lysine, L-arginine or both of these L-amino acids to outside of a cell of a methanol assimilating bacterium when the DNA is introduced into the bacterium, specifically lysE24, is introduced into a methanol assimilating bacterium such as Methylophilus bacteria to improve L-amino acid productivity, especially L-lysine and L-arginine productivities.
Owner:AJINOMOTO CO INC
Non-Transgenic Herbicide Resistant Plants
The present invention relates to the production of a non-transgenic plant resistant or tolerant to a herbicide of the phosphoniorethylglycine family, e.g., glyphosate. The present invention also relates to the use of a recombinagenic oligonucleobase to make a desired mutation in the chromosomal or episomal sequences of a plant in the gene encoding for 5-enol pyruvylshikimate-3-phosphate synthase (EPSPS). The mutated protein, which substantially maintains the catalytic activity of the wild-type protein, allows for increased resistance or tolerance of the plant to a herbicide of the phophonomethylglycine family, and allows for the substantially normal growth or development of the plant, its organs, tissues or cells as compared to the wild-type plant irrespective of the presence or absence of the herbicide. The present invention also relates to a non-transgenic plant cell in which the EPSPS gene has been mutated, a non-transgenic plant regenerated therefrom, as well as a plant resulting from a cross using a regenerated non-transgenic plant having a mutated EPSPS gene.
Owner:CIBUS
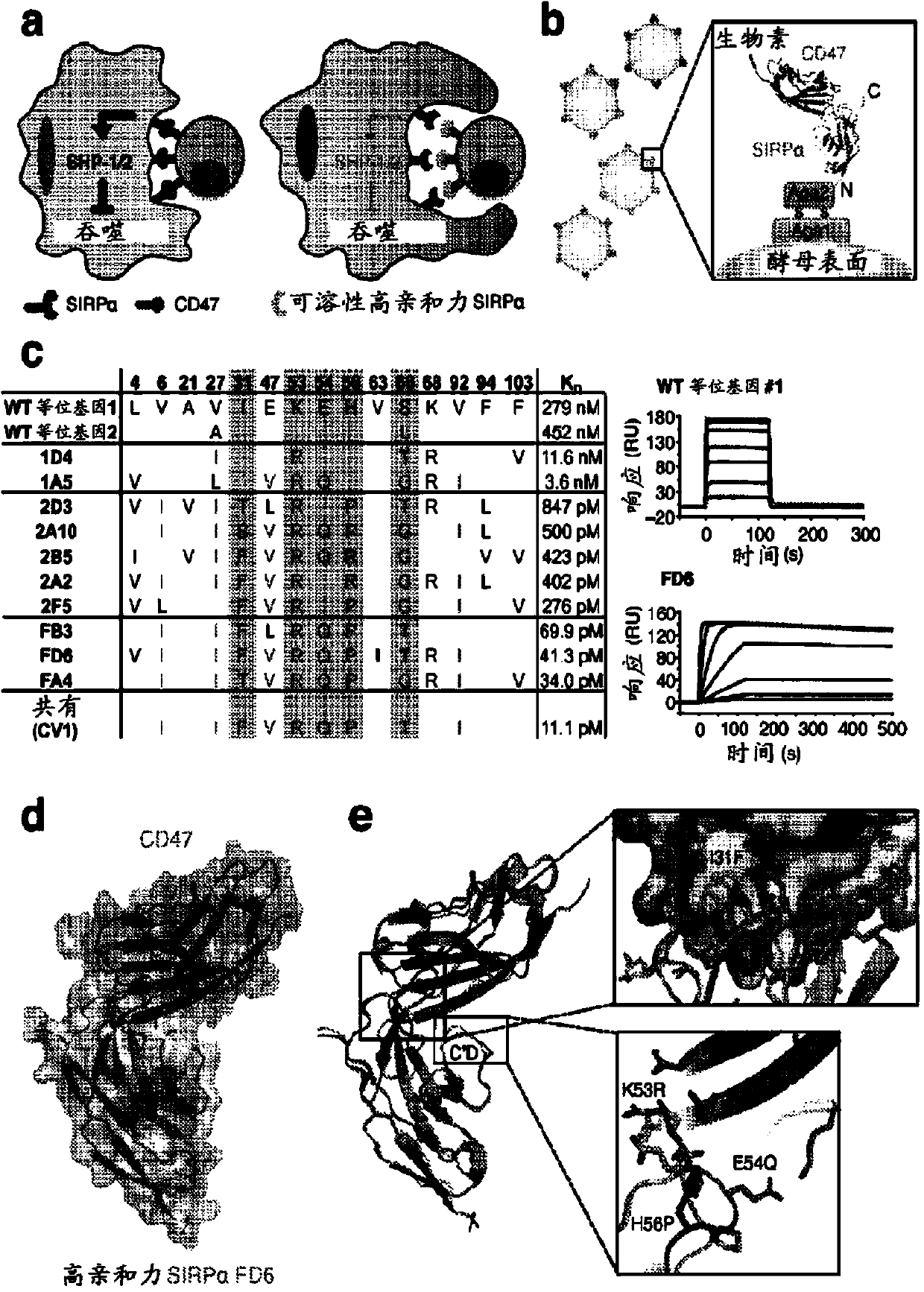
High affinity sirp-alpha reagents
High affinity SIRP-alpha reagent are provided, which (i) comprise at least one amino acid change relative to the wild-type protein; and (ii) have an increased affinity for CD47 relative to the wild-type protein. Compositions and methods are provided for modulating phagocytosis in a mammal by administering a therapeutic dose of a pharmaceutical composition comprising a high affinity SIRPalpha reagent, which blocks the physiological binding interaction between SIRPalpha and its ligand CD47.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
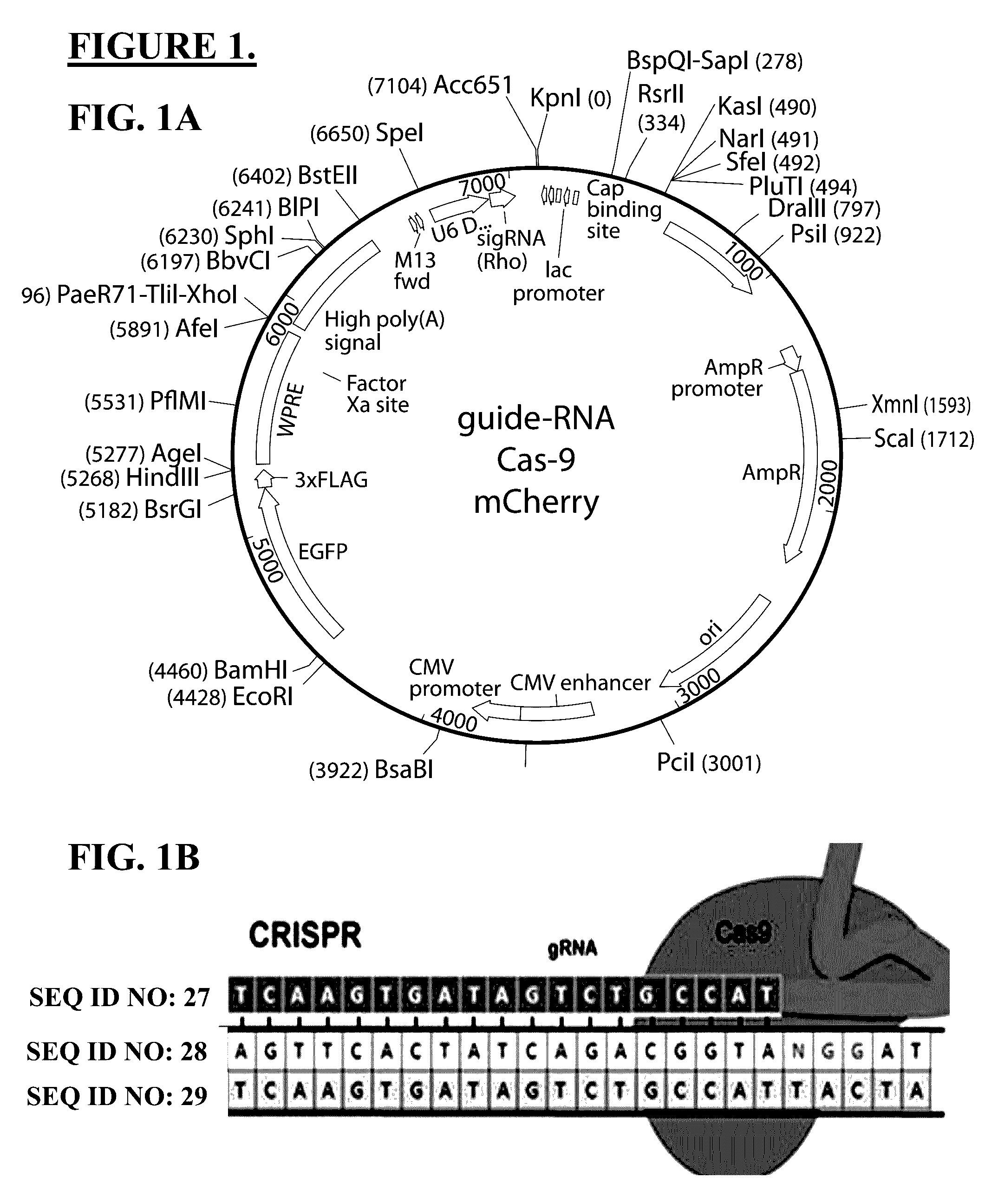
Use of crispr/cas9 as in vivo gene therapy to generate targeted genomic disruptions in genes bearing dominant mutations for retinitis pigmentosa
PendingUS20160324987A1Cell receptors/surface-antigens/surface-determinantsPeptide/protein ingredientsDiseaseIn vivo
Described herein are methods and compositions for genomic editing. Clustered regularly interspaced short palindromic (CRISPR) allows for highly selective targeting and alteration of genetic loci. Here, the Inventors demonstrate CRISPR as capable of being used in living animals to prophylactically prevent a genetic disease from manifesting. Targeting and disruption of mutated rhodopsin gene prevents progression of retinitis pigmentosa in the retinal cells of a transgenic rat model. Such techniques allow for treatment methods in subjects with dominant genetic mutations, often associated with lack of a gene product, or a toxic gene product. The described technology effectively abrogates deleterious effects due to the presence of a mutated gene copy allowing the normal function of the wild-type protein to prevent cell and vision loss. The efficacy of these in vivo mechanisms are widely extensible to similar dominant negative gene mutations causing disease, or other types of genetic disease.
Owner:CEDARS SINAI MEDICAL CENT
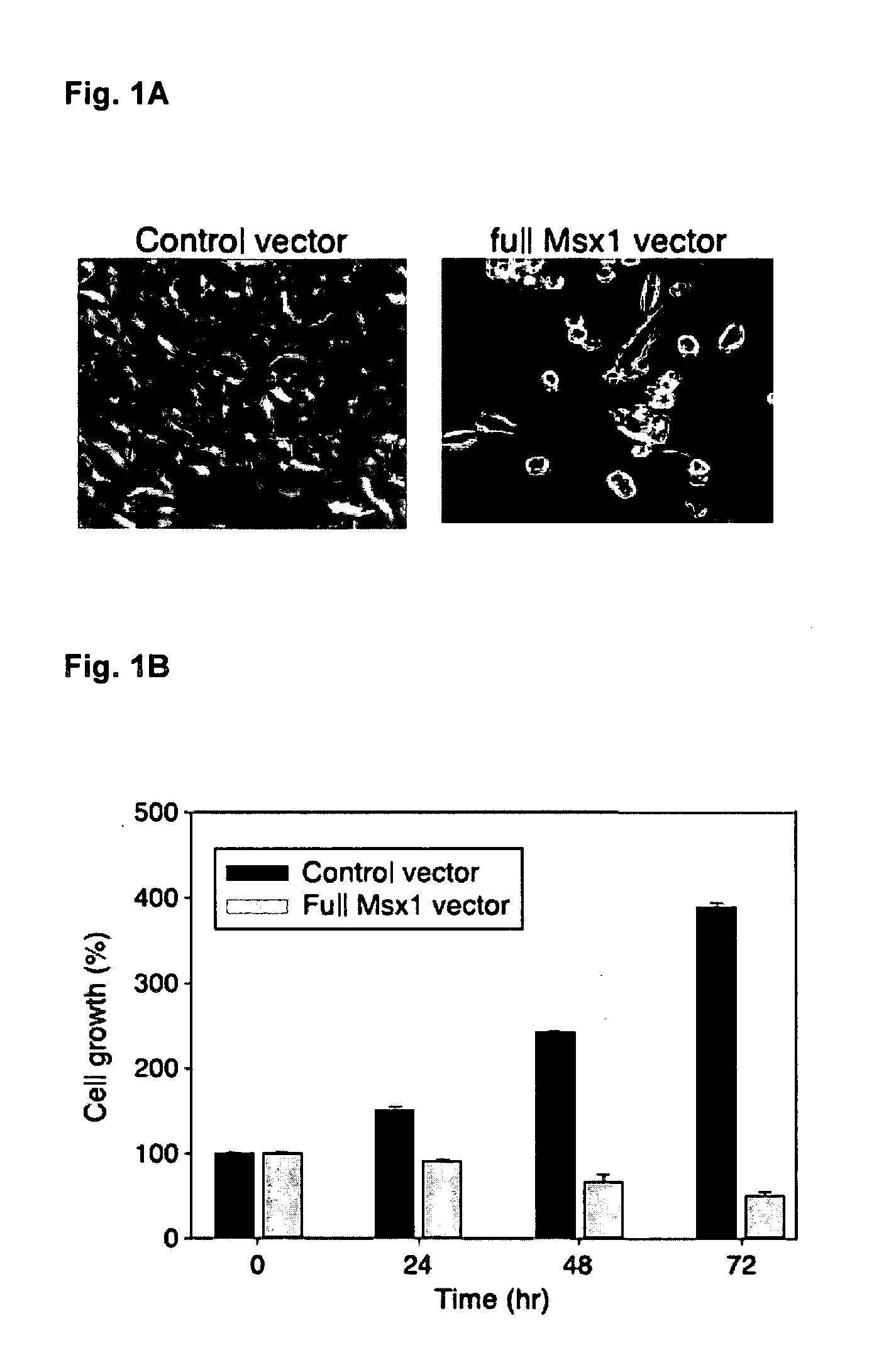
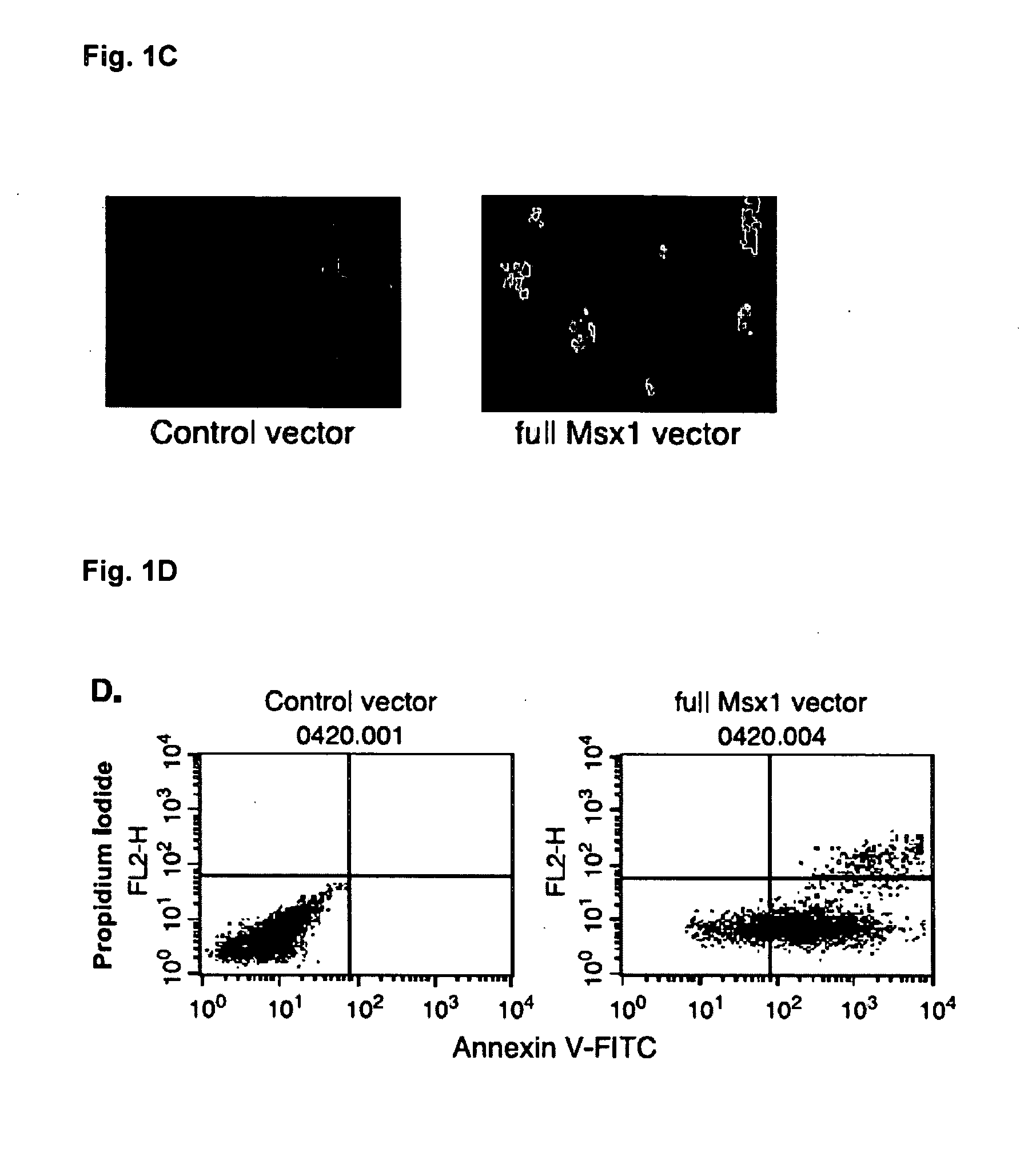
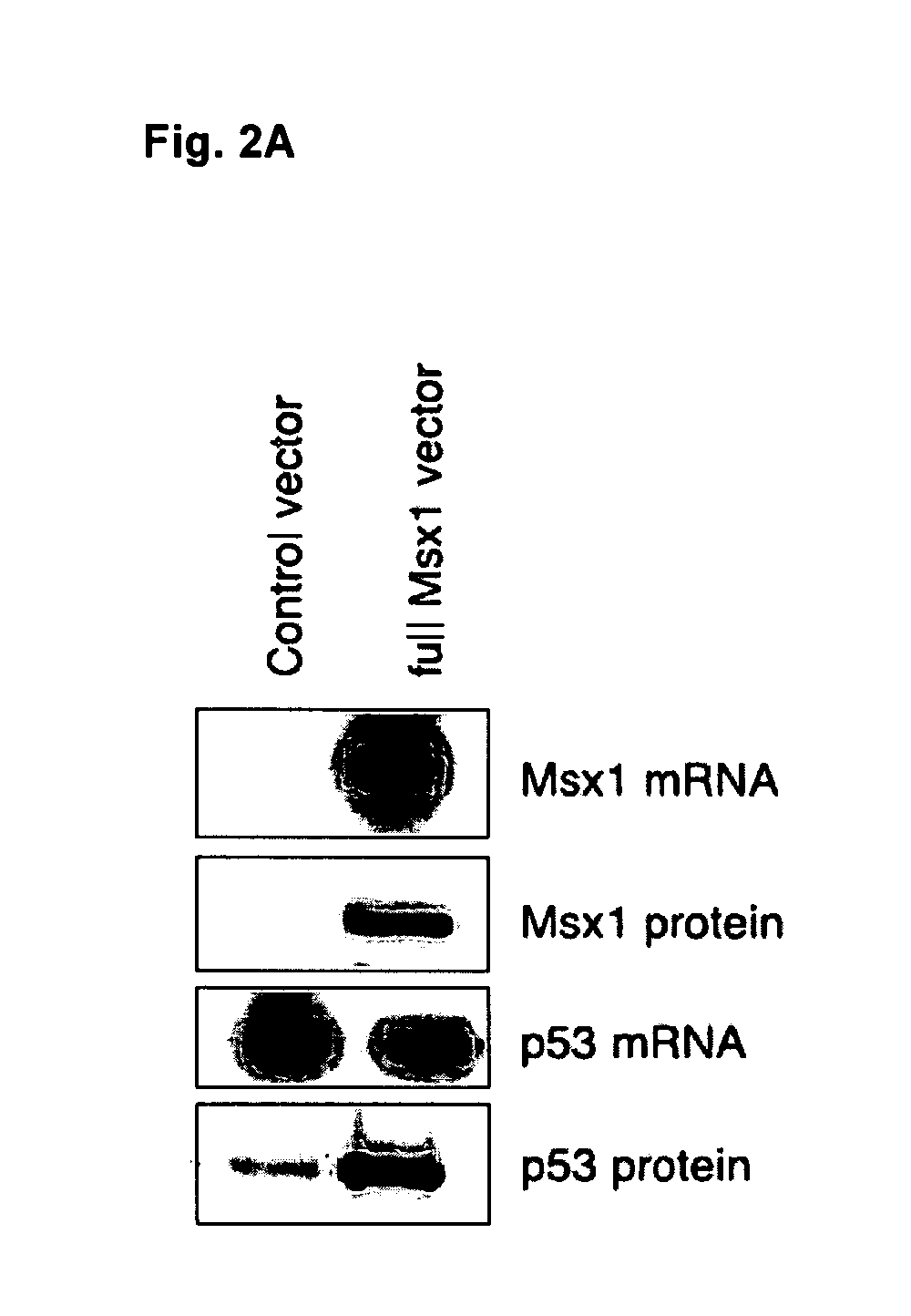
Novel agent for inducing apoptosis comprising Msx1 or a gene encoding the same as an active ingredient
InactiveUS20070021337A1Effectively modulating apoptosisEffectively induce apoptosisPeptide/protein ingredientsEnzymesAbnormal tissue growthBULK ACTIVE INGREDIENT
The present invention relates to a novel use of Msx1 protein or a nucleotide encoding the same for inducing apoptosis. The Msx1 of the present invention induces apoptosis through direct interaction with p53 via a homeodomain and such interaction leads to increased stability, and / or nuclear localization of p53 in cells. The Msx1 or homeodomain thereof can be effectively used for the treatment of tumors, in which wild-type p53 protein has lost its function by some mechanism that inactivates p53 proteins.
Owner:RES & BUSINESS FOUNDATION SUNGKYUNKWAN UNIV
Mutant Pneumolysin Proteins
InactiveUS20080112964A1Low toxicityReduced oligomerisation activityAntibacterial agentsPeptide/protein ingredientsSynechococcusPyrococcus
The invention relates to immunogenic compositions comprising mutant Streptococcus pneumoniae pneumolysin proteins. The invention further relates to such proteins and nucleic acids encoding these proteins. In particular embodiments, the invention is directed to an isolated mutant pneumolysin (PLY) protein, wherein the mutant PLY protein differs from the wild type PLY protein by the presence of a mutation within the region of amino acids 144 to 161 of the wild type sequence, such that the toxicity of the mutant is reduced relative to that of the wild-type protein. In particular embodiments, the mutant PLY protein differs from the wild type protein by the substitution or deletion of amino acids within this region, including the deletion of two adjacent amino acids within the region of amino acids 144 to 151 of the wild type sequence.
Owner:THE UNIV COURT OF THE UNIV OF GLASGOW
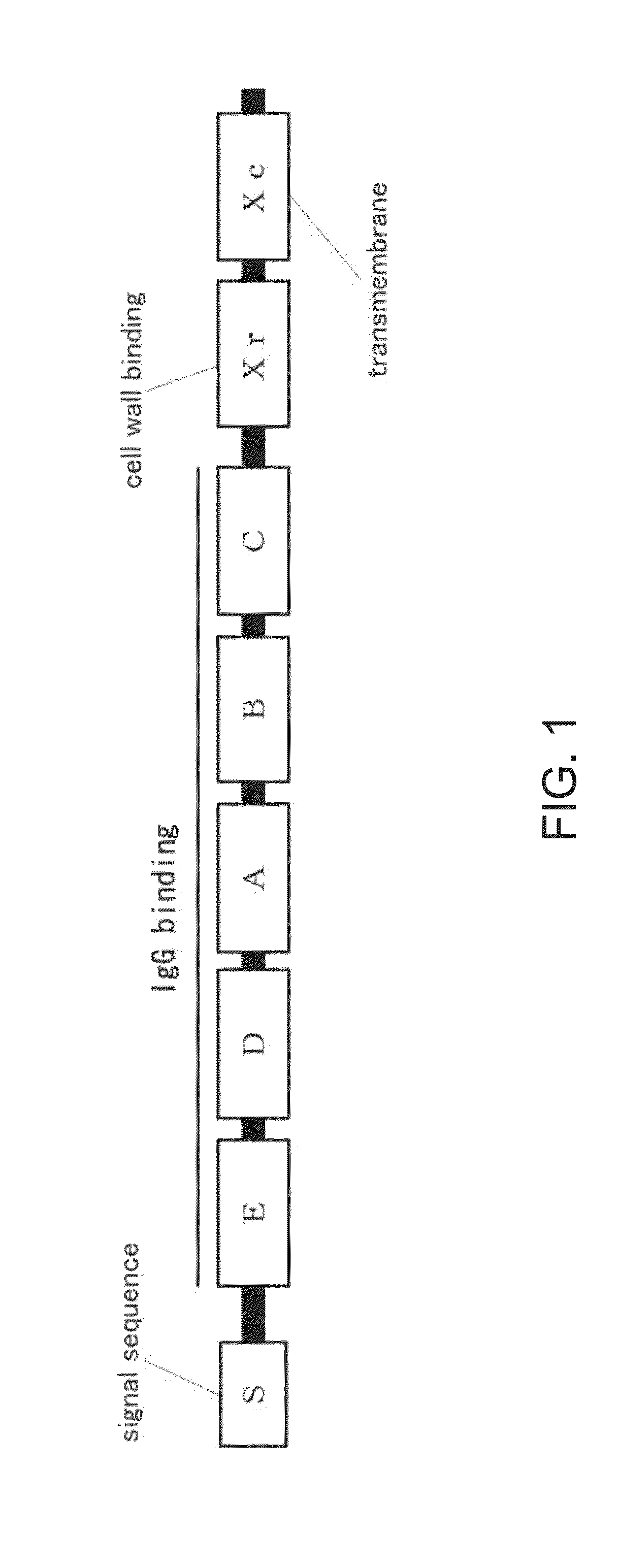
Mutated protein of protein a having reduced affinity in acidic region and antibody-capturing agent
ActiveUS20140179898A1Reduced ability to bindEasy to eluteBacteriaPeptide/protein ingredientsMutated proteinProteome
A modified protein of an extracellular domain of protein A, which has the reduced ability to bind to immunoglobulin in an acidic region, compared with the wild-type extracellular domain of protein A, without impairing a selective antibody-binding activity in a neutral region. On the basis of three-dimensional structure coordinate data on a complex of the extracellular domain of protein A bound with the Fc region of immunoglobulin G, the modified protein is obtained by the substitution of amino acid residues that are located within the range of 10 angstroms from the Fc region and have a 20% or more ratio of exposed surface area, by histidine residues. Preferably, the modified protein is obtained by the substitution of amino acid residues at sites identified from the analysis of sequences selected from a library constituted by the protein group, by histidine residues. These substitutions may be combined.
Owner:NAT INST OF ADVANCED IND SCI & TECH
Method for stabilization of proteins using non-natural amino acids
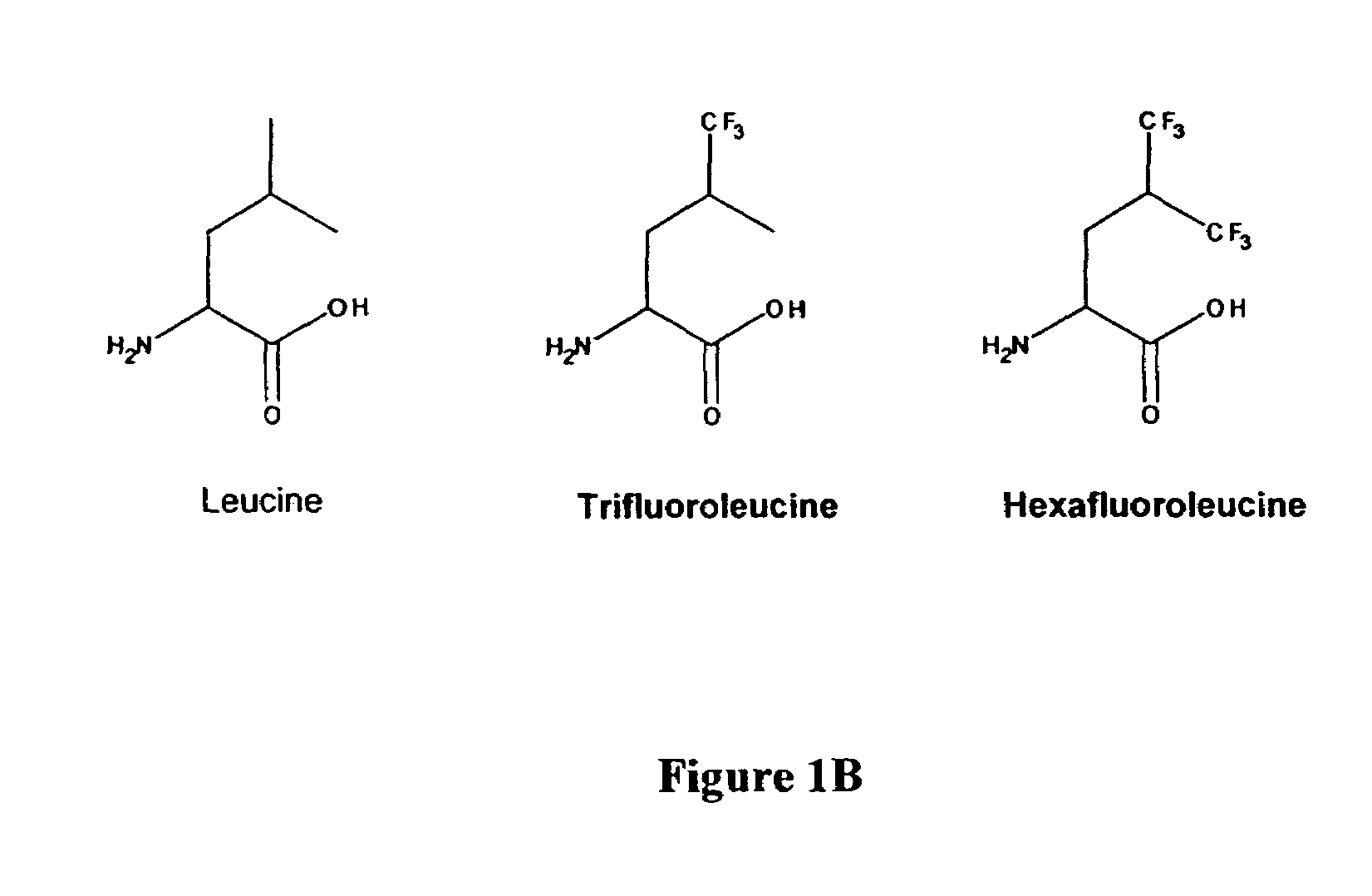
InactiveUS7449443B2Peptide/protein ingredientsProtein composition from yeastsCoiled coil proteinTert-leucine
The present invention provides a method for producing modified stable polypeptides introducing at least one non-natural amino acid into the hydrophobic region of the polypeptide. The thermal and chemical stability of such polypeptides is improved compared to those properties of its corresponding wild type proteins.The invention further provides purified leucine zipper and coiled-coil proteins in which the leucine residues have been replaced with 5,5,5-trifluoroleucines, and the modified proteins so produced demonstrate increased thermal and chemical stability compared to their corresponding wild-type natural proteins.
Owner:CALIFORNIA INST OF TECH
Transgenic macrophages, chimeric antigen receptors, and associated methods
ActiveUS10415017B2Cell receptors/surface-antigens/surface-determinantsAntibody mimetics/scaffoldsCytoplasmic partADAMTS Proteins
Described herein are chimeric receptors. Chimeric receptors comprise a cytoplasmic domain; a transmembrane domain; and an extracellular domain. In embodiments, the cytoplasmic domain comprises a cytoplasmic portion of a receptor that when activated polarizes a macrophage. In further embodiments, a wild-type protein comprising the cytoplasmic portion does not comprise the extracellular domain of the chimeric receptor. In embodiments, the binding of a ligand to the extracellular domain of the chimeric receptor activates the intracellular portion of the chimeric receptor. Activation of the intracellular portion of the chimeric receptor may polarize the macrophage into an M1 or M2 macrophage.
Owner:THUNDER BIOTECH INC
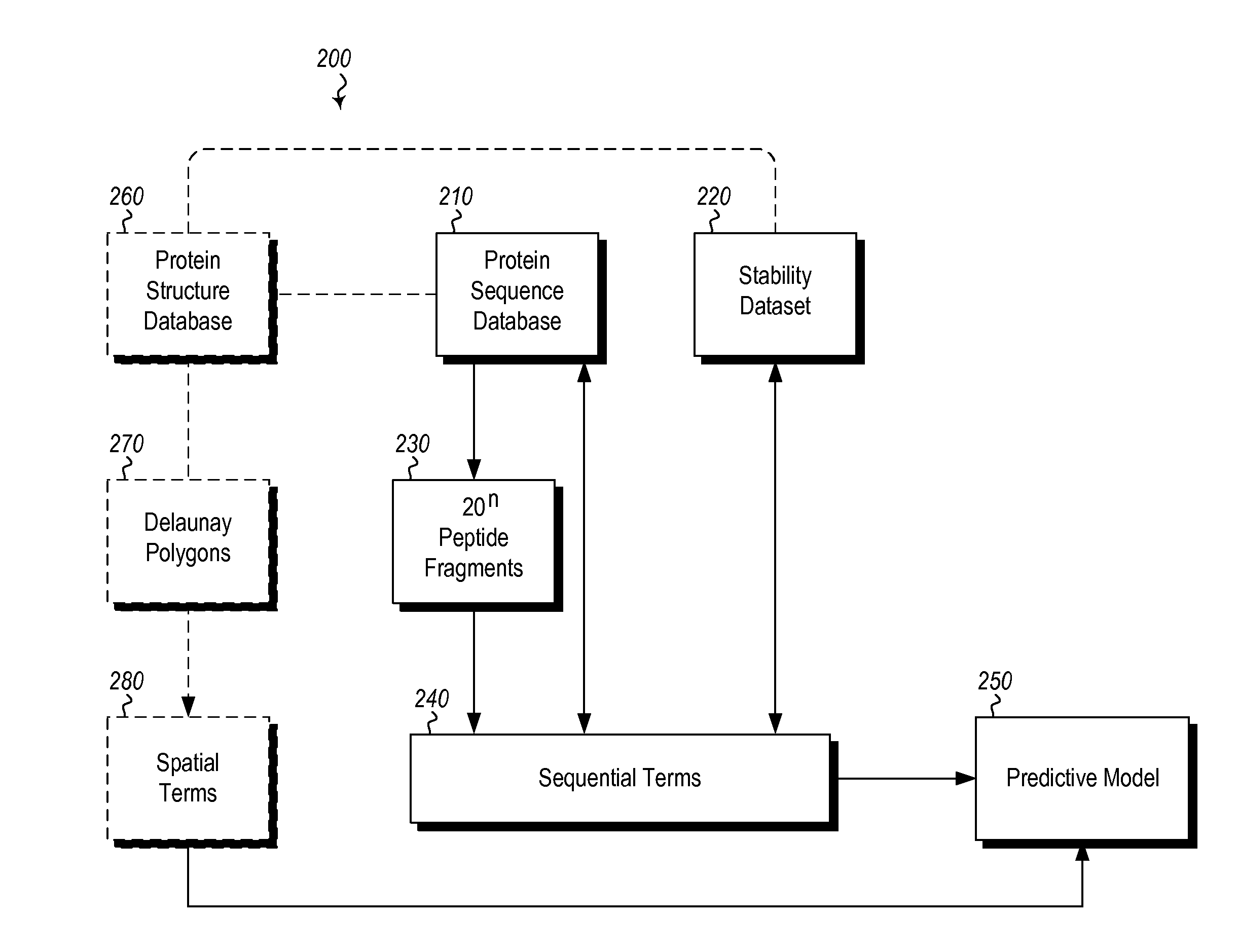
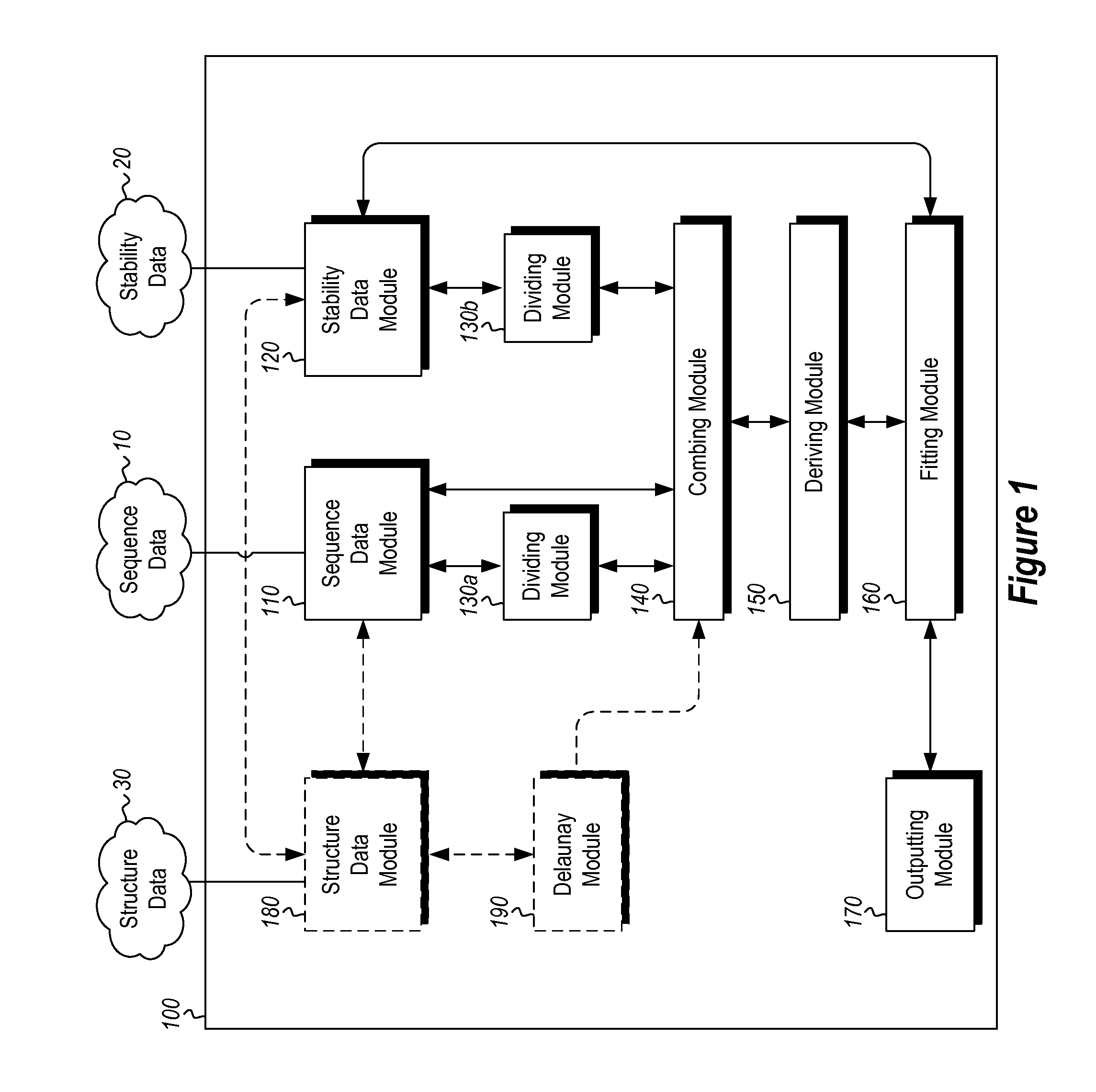
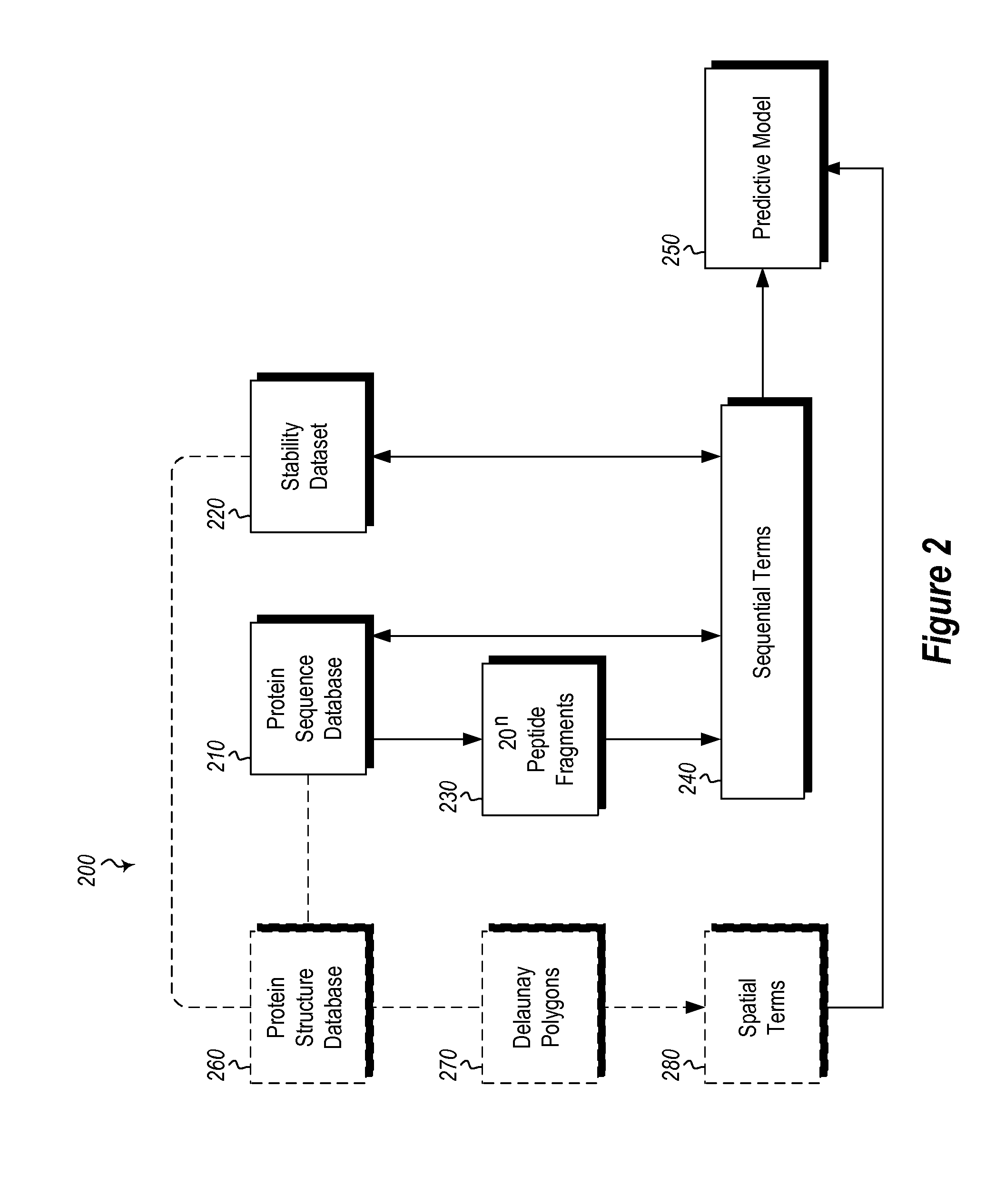
Methods and systems for designing stable proteins
InactiveUS20120265513A1Improve protein stabilityImprove thermal stabilityAnalogue computers for chemical processesProteomicsProtein structureThermophilic organism
Methods and computing systems for generating a protein stability lookup table and a predictive model. These methods and systems are useful for predicting the thermal stability of a protein sequence and for predicting mutations that may enhance the thermal stability of a protein given its amino acid sequence and / or three dimensional structure. The protein stability lookup table and a predictive model are based on a combination and analysis of related protein sequences and, where available, protein structure data, and relative stability data from mesophilic and thermophilic organisms and experimentally determined stability changes of wild type proteins and their mutants.
Owner:UNIVERSITY OF KANSAS
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com