Application of FDPS (farnesyl pyrophosphate synthase) for preparing medicine capable of treating nonalcoholic steatohepatitis
A steatohepatitis and non-alcoholic technology, which is applied in the direction of drug combination, microbial measurement/testing, and pharmaceutical formulations, can solve the problems of lack of in-depth research in application, and achieve the effect of reducing liver lipid deposition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
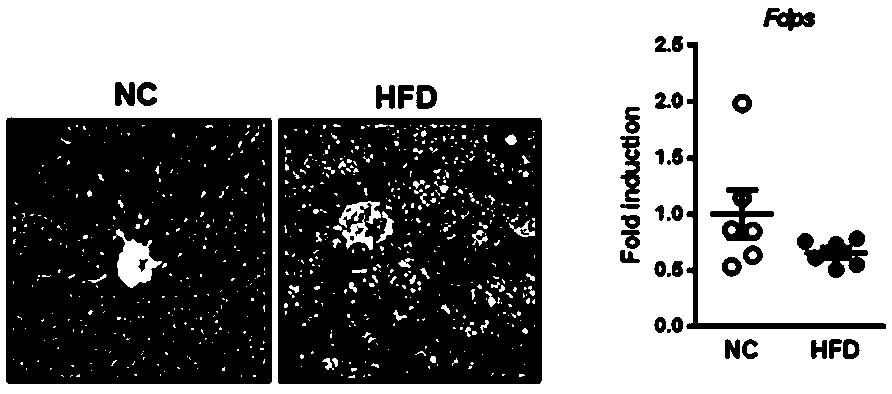
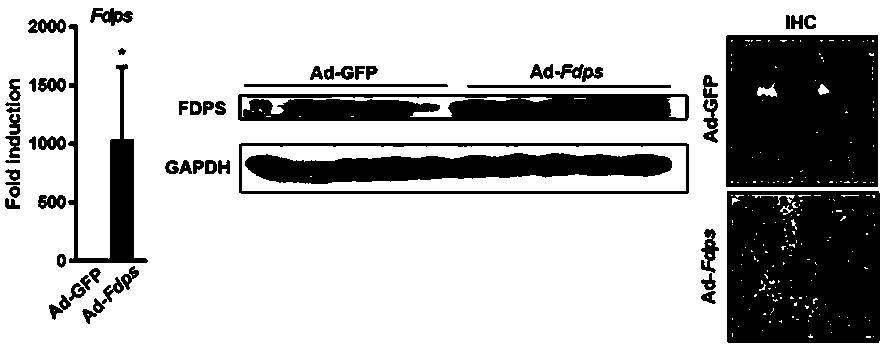
[0035] FDPS is a potential target for the treatment of NASH
[0036] 1. Construction of NASH model:
[0037] Model 1: 5-week-old male C57BL / 6J mice (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.) were raised in a standard SPF environment (no special pathogens, constant temperature at 25°C, and lighting at intervals of 12 hours). The mice were divided into two groups, a group of mice (n=6) were fed with maintenance feed (NC), and a group of mice (n=6) were fed with high-fat diet (HFD) (the high-fat feed was purchased from the American Research Diet company, Cat. No. D12492). After 21 weeks, the mice were anesthetized with chloral hydrate. The mice were dissected, and the plasma was collected. One lobe of the liver was quick-frozen in liquid nitrogen, stored at ultra-low temperature, and one lobe of the liver was fixed in formalin.
[0038] Model 2: 5-week-old male C57BL / 6J mice (purchased from Beijing Weitong Lihua Experimental Animal Technolog...
Embodiment 2
[0103] The therapeutic effect of alendronate sodium on NASH
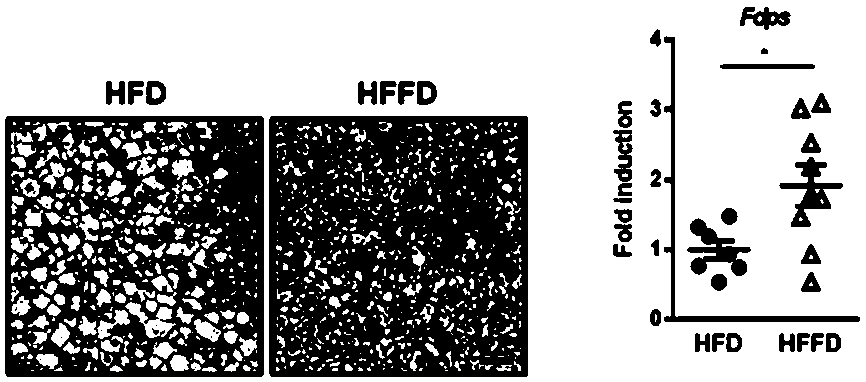
[0104] Construction of HFFD model: 5-week-old male C57BL / 6J mice (purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd.) were bred in a standard SPF environment (constant temperature 25°C, lighting intervals of 12 hours). During modeling, they were fed with high-fat feed and water containing fructose (2.31g fructose / 100mL drinking water) (HFFD), free to eat and drink.
[0105] Alendronate sodium administration regimen: divided into groups after feeding for 10 weeks, solvent control group (Control / Ctrl) (n=8) and alendronate sodium administration group (ALN) (purchased from Apexbio, USA, product number B6586) (n=9). On every Monday, Wednesday, and sixth day, the mice in the control group and the alendronate sodium administration group were injected intraperitoneally with equal volumes of normal saline and alendronate sodium solution (1 mg / kg, alendronate sodium solid powder for physiological ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com