Benzoyl-modified aggregation-induced light-emitting aniline oligomer and preparation method thereof
A technology of aggregation-induced luminescence and oligomers, which is applied in the preparation of carboxylic acid amides, organic compounds, luminescent materials, etc., to achieve the effects of simple operation steps, reduced error probability, and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
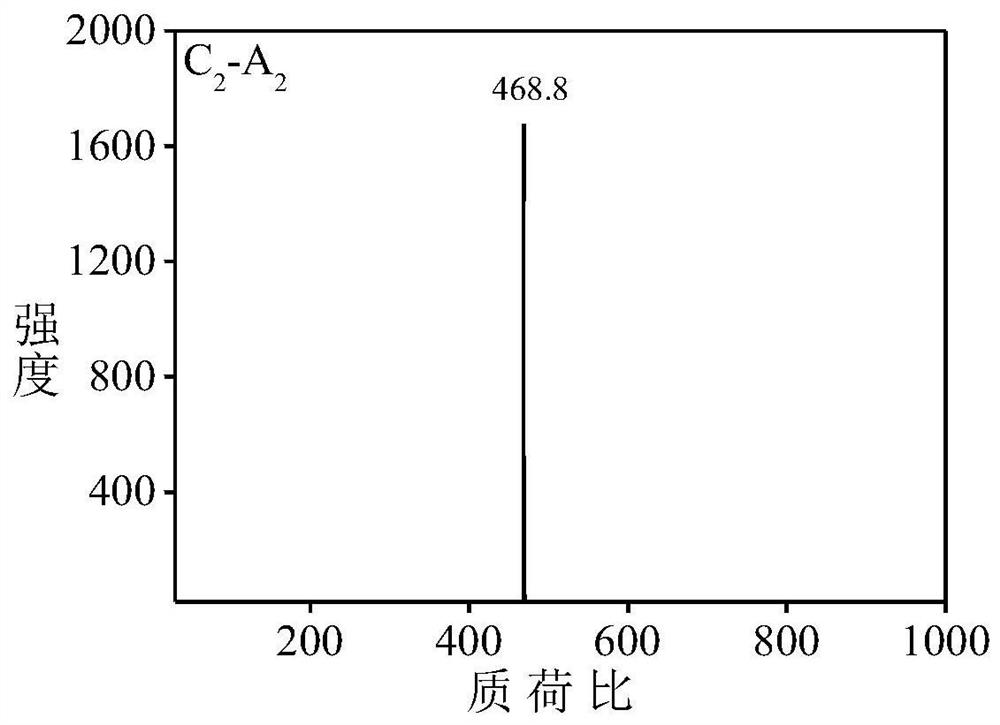
[0051] Aniline dimer derivative C 2 -A 2 preparation of
[0052] Aniline dimer derivative C 2 -A 2 The synthetic route of is as follows:
[0053]
[0054] Specifically include the following steps:
[0055] Step 101: Weigh 1g of N,N'-diphenyl-1,4-p-phenylenediamine and place it in a 100ml three-neck flask, add 3ml of pyridine as an acid-binding agent and 20ml of chloroform as a solvent to fully dissolve to obtain a solution ;
[0056] Step 102: Measure 6ml of benzoyl chloride, dissolve it in 15ml of chloroform and dilute it, slowly add it dropwise to the flask in an ice-water bath and stir evenly. After the dropwise addition, move the device to room temperature for 4 hours of reaction. Spot the plate to monitor the progress of the reaction. After the reaction, a brown-red solution was obtained.
[0057] Step 103: After the reaction is finished, filter the filtrate, dilute the filtrate with chloroform, wash with hydrochloric acid aqueous solution to remove excess p...
Embodiment 2
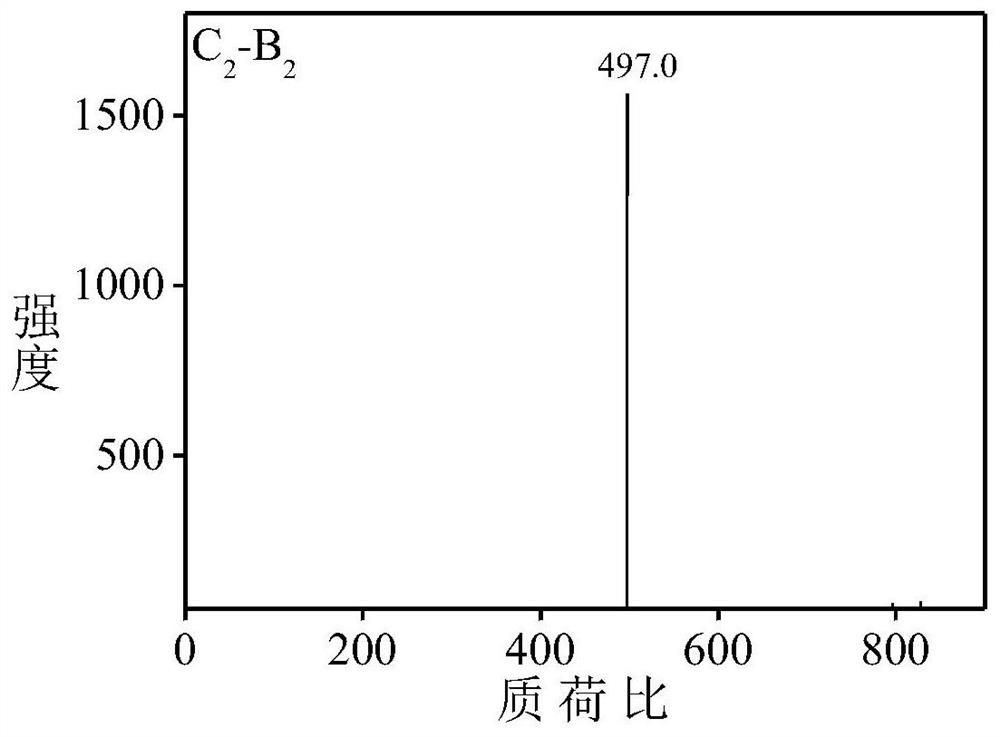
[0059] Aniline dimer derivative C 2 -B 2 preparation of
[0060] Aniline dimer derivative C 2 -B 2 The synthetic route of is as follows:
[0061]
[0062] Specifically include the following steps:
[0063] Step 201: Weigh 1g of N,N'-diphenyl-1,4-p-phenylenediamine and place it in a 100ml three-neck flask, add 3ml of pyridine as an acid-binding agent and 25ml of chloroform as a solvent to fully dissolve, and obtain a solution ;
[0064] Step 202: Measure 6ml of phenylacetyl chloride, dissolve it in 10ml of chloroform and dilute it, slowly drop it into the flask in an ice-water bath and stir evenly. The board monitors the progress of the reaction;
[0065] Step 203: After the reaction is over, filter the filtrate, extract with excess chloroform and hydrochloric acid aqueous solution, and then repeatedly wash with aqueous solution until the pH is about 7 to remove excess pyridine and pyridine hydrochloride; separate the organic layer and add appropriate amount of Wa...
Embodiment 3
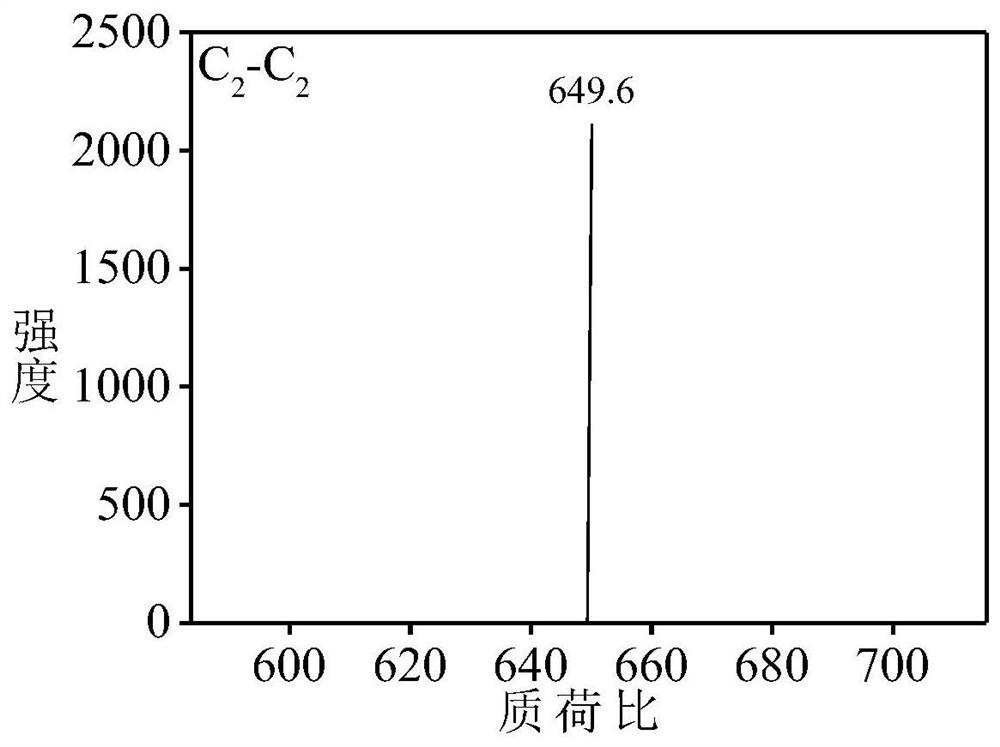
[0067] Aniline dimer derivative C 2 -C 2 preparation of
[0068] Aniline dimer derivative C 2 -C 2 The synthetic route of is as follows:
[0069]
[0070] Specifically include the following steps:
[0071] Step 301: Weigh 1g of N,N'-diphenyl-1,4-p-phenylenediamine and place it in a 100ml three-neck flask, add 3ml of pyridine as an acid-binding agent and 25ml of dichloromethane as a solvent to fully dissolve, and obtain a solution ;
[0072] Step 302: Measure 2.6ml of diphenylacetyl chloride, dissolve it in 10ml of dichloromethane and dilute it, slowly add it dropwise to the flask in an ice-water bath and stir evenly, after the drop is completed, move the device to room temperature for 4 hours to react. During the process, the plate is continuously tapped to monitor the reaction process;
[0073] Step 303: After the reaction is completed, filter the filtrate, extract with excess dichloromethane and hydrochloric acid aqueous solution, and then repeatedly wash with a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com