Anti-aggregation-caused-quenching functional heptamethine cyanine dye based on click activation large steric hindrance as well as preparation method and application thereof
An alkyl and aryl technology, applied in biomedical imaging, anti-aggregation and quenching heptamethasone functional cyanine dye and its preparation field, can solve the problem of low background signal-to-noise ratio, improve detection sensitivity, Fast and sensitive response ability, increase the effect of fluorescent signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] The synthetic route of diethylene glycol bromoethyl methyl ether parent dye CyBI7P:
[0069]
[0070] 1.1 Synthesis of diethylene glycol bromoethyl methyl ether benzindole quaternary ammonium salt:
[0071] Dissolve 0.611g of 1,1,2-trimethyl 1[H]benzindole, 0.917g of diethylene glycol-2-bromoethyl methyl ether in 10ml of acetonitrile, and react the mixture in a reaction kettle at 80°C for 36 hours , standing to cool, then poured into ether for washing, filtered, and recrystallized from methanol to obtain 0.96 g of diethylene glycol bromoethyl methyl ether benzindole bromide quaternary ammonium salt, with a yield of 80%.
[0072] 1.2 Synthesis of condensing agent 2-chloro-1-formyl-3-hydroxymethinecyclohexene:
[0073] 18ml POCl 3 Add 17ml of dry dichloromethane solution and mix for later use. Add 40ml of dry dichloromethane and 40ml of dry DMF in turn to a 250mL round bottom flask and mix well. Under ice bath conditions, the mixed POCl 3 The dichloromethane solut...
Embodiment 2
[0077] Synthesis of CyP-Tz, a functionalized Anti-ACQ heptamethine cyanine dye with click activation:
[0078]
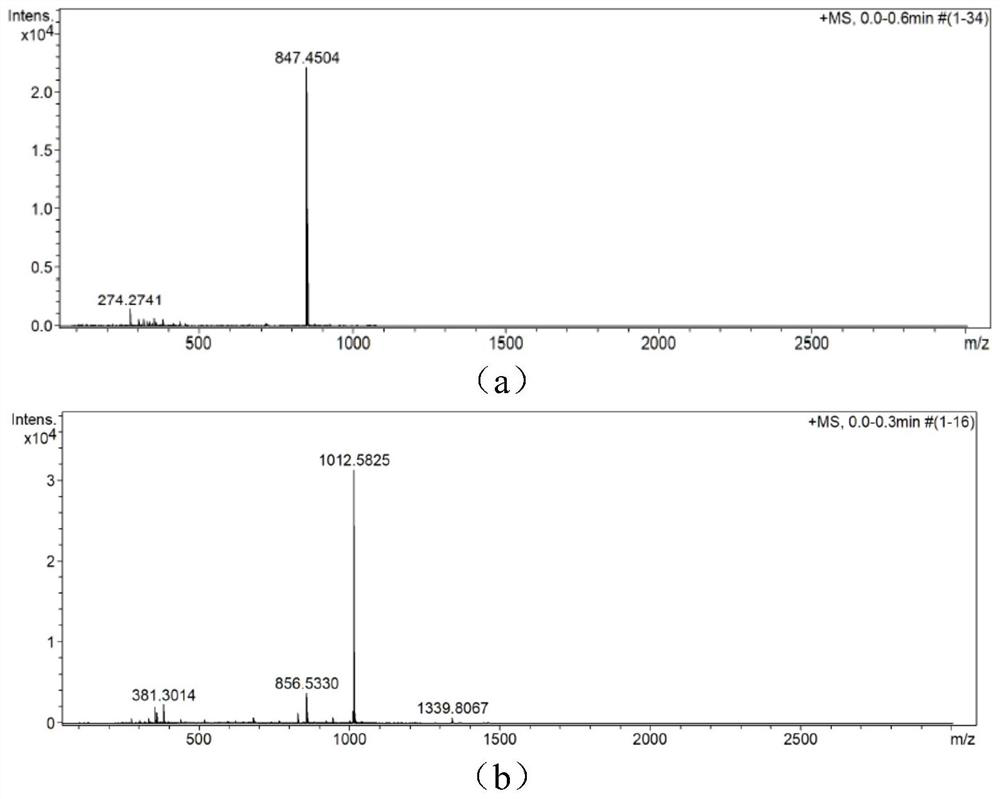
[0079] The parent dye CyBI7P prepared in Example 1 was pre-dried in a vacuum oven at 40° C. overnight. Add dry 0.5g CyBI7P and 0.51g Tz in turn to a 100ml round bottom flask, add 15ml anhydrous DMF to dissolve. After stirring for 1.5 h at 80°C under the protection of nitrogen, the reaction solution was quenched by pouring into ice water, and a blue solid was precipitated by filtration, separated by column chromatography (methanol:dichloromethane=1:50v / v), and concentrated with petroleum ether to precipitate a solid , vacuum dried overnight to obtain the blue dye CyP-Tz 0.3g, yield 50.92%. The product structure is identified by high resolution. Calculated value: 1012.5701, Tested value: 1012.5843. Mass spectrometry results such as figure 1 Shown in (b).
Embodiment 3
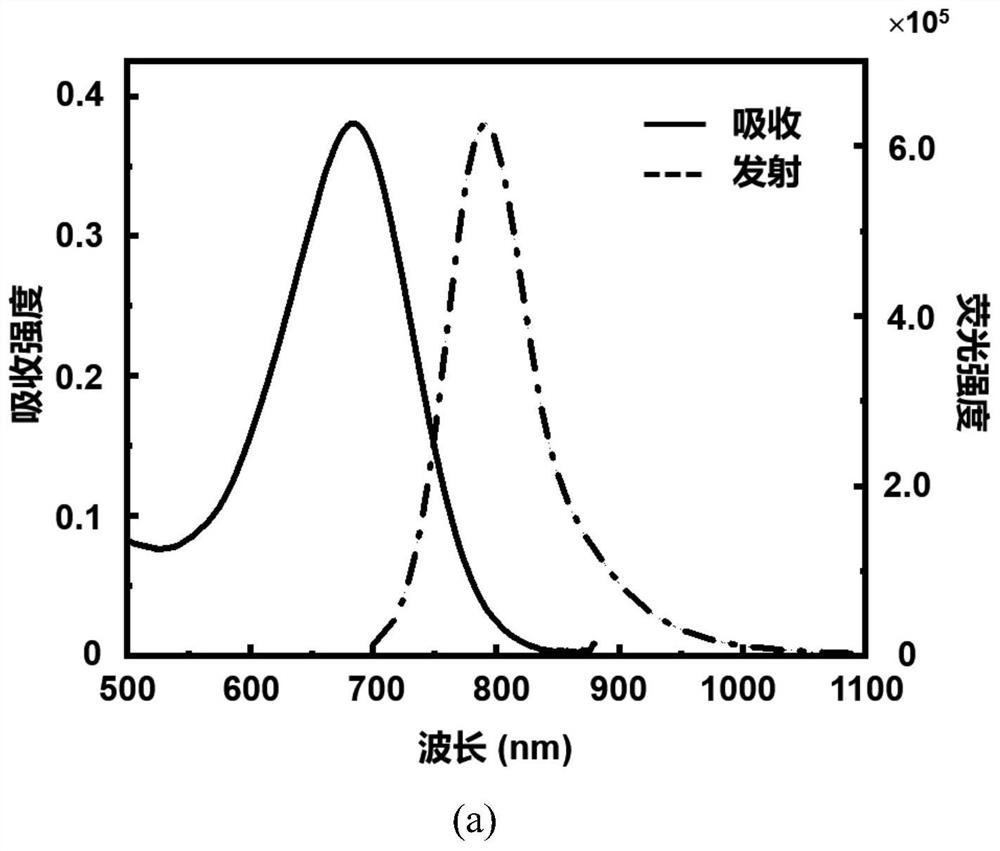
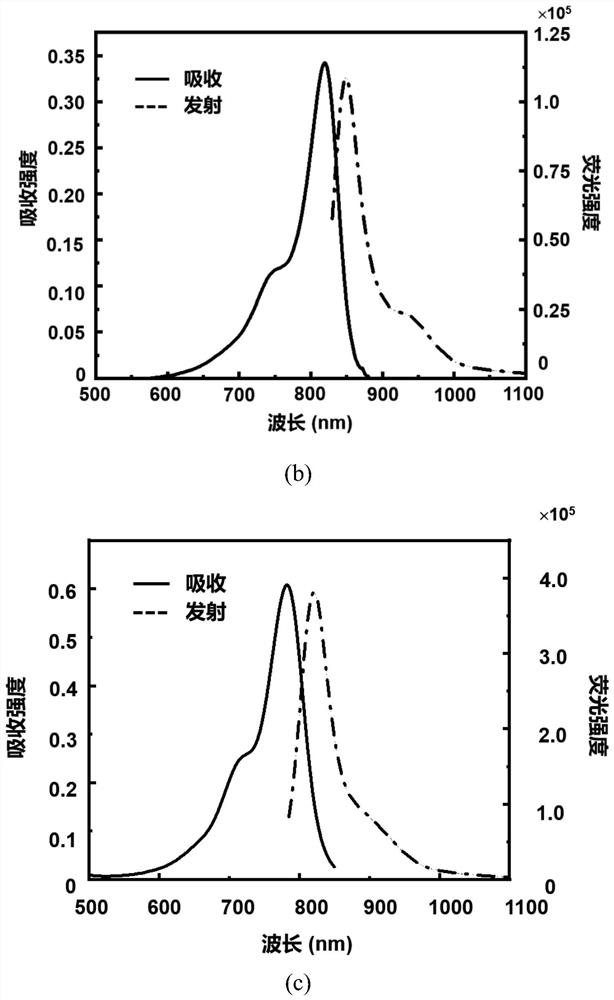
[0081] The spectral characteristics of the click-activated mid-position-substituted functionalized Anti-ACQ heptamethine cyanine dye CyP-Tz, the parent dye CyBI7P, and the commercial heptamethine cyanine dye ICG (CAS: 3599-32-4):
[0082] Accurately weigh 5 mg of CyP-Tz solid with a balance, dissolve it in 1 ml of dimethyl sulfoxide (DMSO) to prepare a mother solution with a concentration of 4.58 mM; then take the mother solution and dilute it in methanol to obtain a test solution. The absorption intensity and fluorescence emission intensity of CyP-Tz were measured by UV spectrophotometer and Edinburgh 1000 fluorescence spectrometer. The molar absorptivity of the compounds was calculated using the Lambert-Beer law. The calculation formula is A=εcb, where A represents the absorption intensity, ε represents the molar extinction coefficient, c is the concentration of the compound (in mol / L), and b is the thickness of the quartz cell (in cm). The test result shows that the UV abs...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com