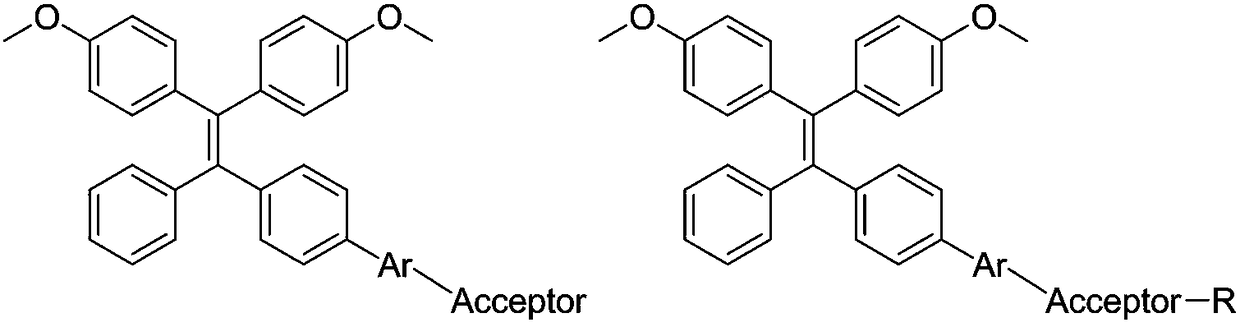
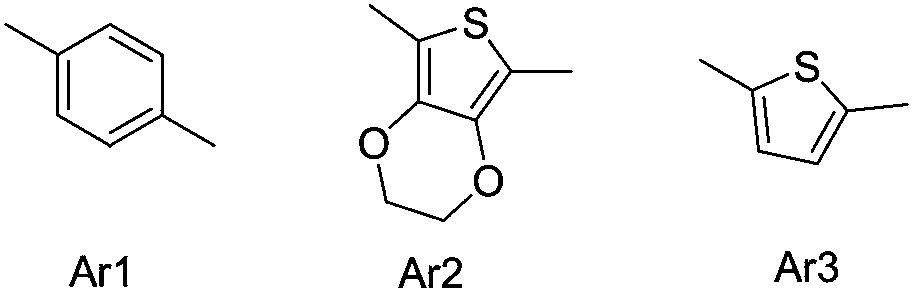
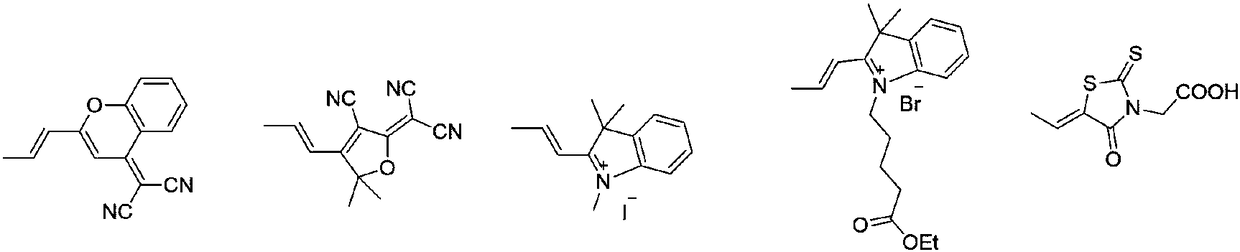
Novel fluorescent dye synthetic method with aggregation-induced emission phenomenon
A technology of aggregation-induced luminescence and fluorescent dyes, applied in the field of high-performance functional compounds and their preparation, can solve the problems of unfavorable fluorescence sensing and imaging, improving the difficulty of biomolecular tracking analysis, and reducing the sensitivity of probes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] (E)-2-((2-(2-(5-((4'-(2,2-bis(4-methoxyphenyl)-1-phenylvinyl)-[1,1'-biphenyl]-4-yl Synthesis of )methylene)-4-oxo-2-thioxothiazolidin-3-yl)acetamido)ethyl)disulfanyl)ethylnitrate
[0027]
[0028] (1) Synthetic intermediate
[0029] 4,4'-(2-(4-bromophenyl)-2-phenylethene-1,1-diyl)bis(methoxybenzene) [1a]:
[0030] Suspend 24.2g (372mmol) of zinc powder in 250mL of tetrahydrofuran (THF) into a 1L three-necked flask, stir with a magnet and blow in argon. The system was lowered to 0°C and 21mL (186mmol) of titanium tetrachloride was added dropwise. After the dropwise addition, the system was returned to room temperature and reacted for 30min, then heated in an oil bath to 70°C for 3h. Then it was lowered to 0°C again, and 7.4g (93mmol) of pyridine was added dropwise first, and then 9g (37.2mmol) of 4,4'-dimethoxybenzophenone dissolved in 200mL tetrahydrofuran (THF) and 4-bromo 12.6 g (48.4 mmol) of benzophenone, after the dropwise addition, the temperature of the oil...
Embodiment 2
[0061] (E)-2-(2-(2-(7-(4-(2,2-bis(4-methoxyphenyl)-1-phenylvinyl)phenyl)-2,3-dihydrothieno[3,4-b][ 1,4]dioxin-5-yl)vinyl)-4H-chromen-4-ylidene)malononitrile synthesis:
[0062]
[0063](1) Synthetic intermediate 2,3-dihydrothieno[3,4-b][1,4]dioxine-5-carbaldehyde [2a]:
[0064] Dissolve 5g (35.21mmol) of 3,4-ethylenedioxythiophene in 70mL of N,N-dimethylformamide (DMF) into a two-neck flask, stir with a magnet and blow in argon. The reaction system was lowered to 0° C., and 10.8 g (70.42 mmol) of phosphorus oxychloride was added dropwise to the system. The system was slowly restored to room temperature for 8 hours overnight. After the reaction is complete, use a rotary evaporator to spin dry the DMF, lower the system to 0°C and add 50 mL of saturated sodium bicarbonate solution to quench the excess phosphorus oxychloride, use 3*50 mL of dichloromethane to extract and separate the liquid, and spin dry the organic Mutually. The sample was mixed with silica gel and passed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com