Acylphloroglucinol derivative as well as pharmaceutical composition and application thereof
A technology for acyl phloroglucinol and derivatives, which is applied in the field of medicine, can solve the problems such as no dearylation prenyl phloroglucinol derivative compound activity, etc., and achieves the effect of convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
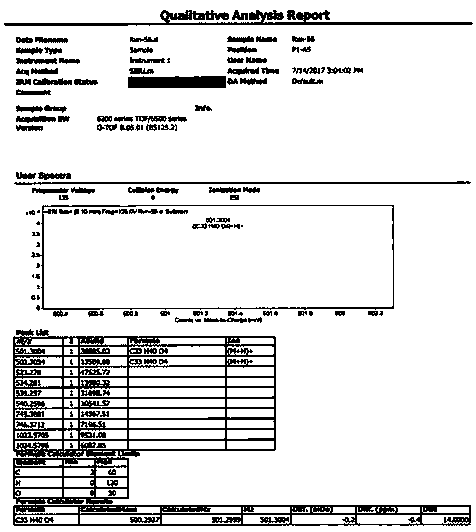
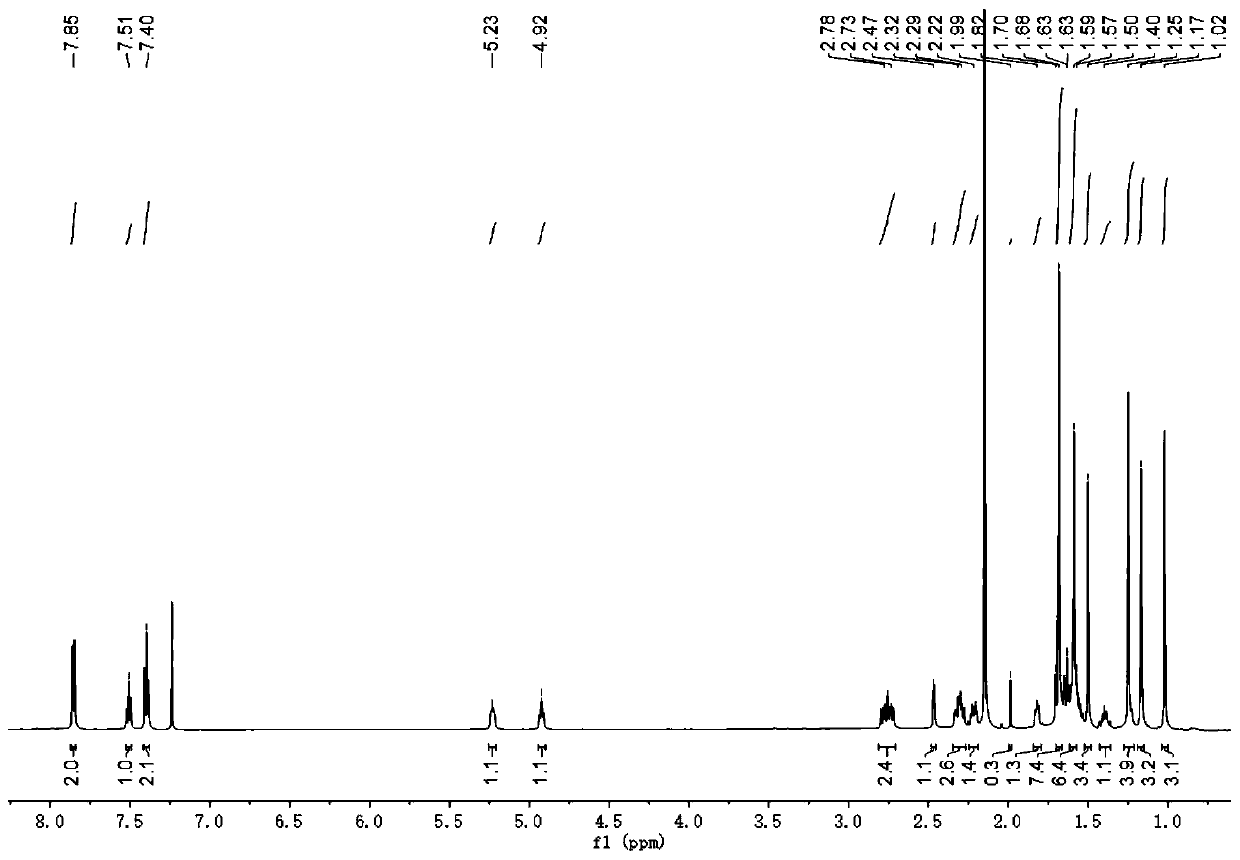
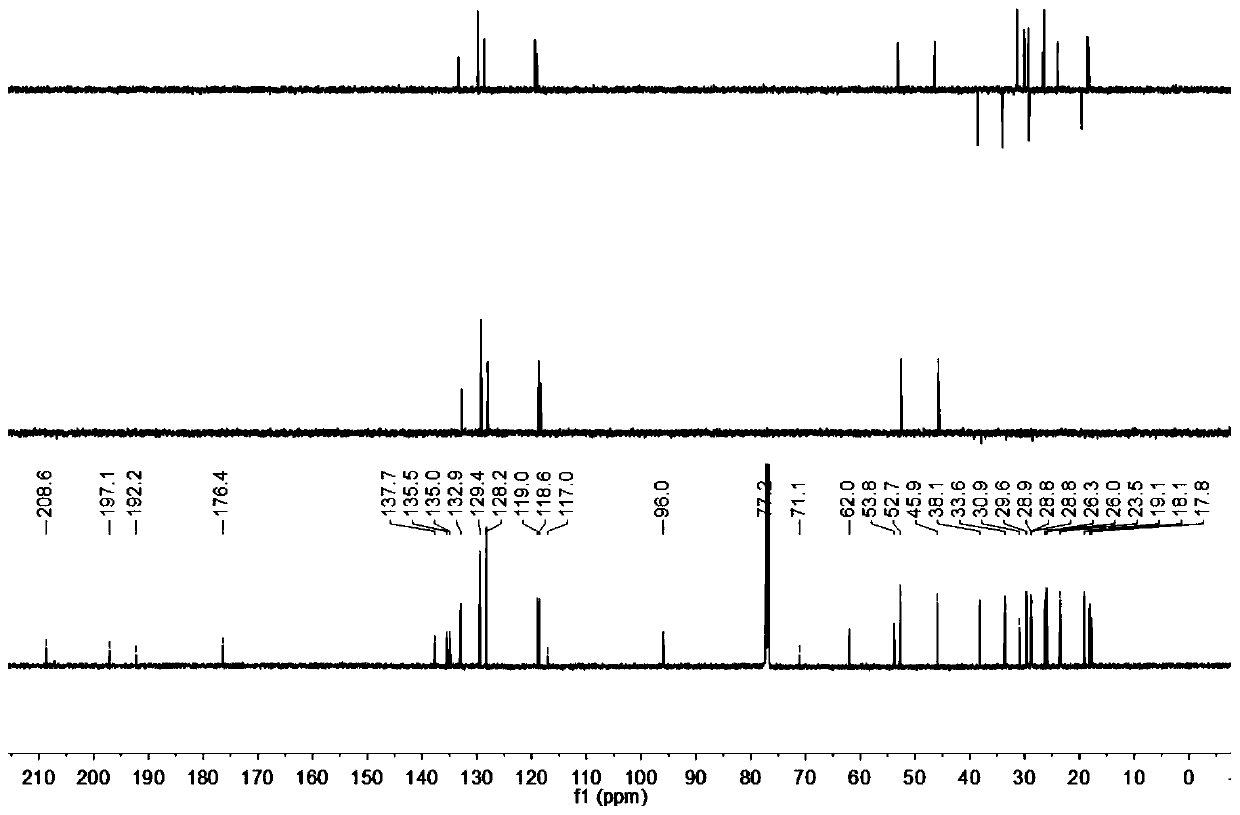
[0027] The present invention provides a preparation method of the compound hypatone A described in the above technical scheme, comprising the following steps: extracting the dried Rhizoma chinensis (12kg) with methanol for 3 times, each time for 2 days, and obtaining the obtained extract by distillation under reduced pressure Extraction, mix the extract with 100-200 mesh silica gel, carry out silica gel column chromatography, the eluent used is chloroform, obtain chloroform section, then mix the obtained chloroform section with polyamide, and use 70%- 100% methanol-water was used as the mobile phase to pass through the MCI column, and five components A to E were collected sequentially. The A (16.4g) component was subjected to silica gel column chromatography under gradient elution conditions, using petroleum ether / acetone as the The eluent was collected in sequence to obtain 5 fractions, which were denoted as fractions A1-A5; fraction A2 was separated and purified by HPLC to ob...
Embodiment 1
[0037] The preparation method of compound hypotone A, comprises the following steps:
[0038] The present invention provides a preparation method of the compound hypatone A described in the above technical scheme, comprising the following steps: extracting the dried Rhizoma chinensis (12kg) with methanol for 3 times, each time for 2 days, and obtaining the obtained extract by distillation under reduced pressure Extraction, mix the extract with 100-200 mesh silica gel, carry out silica gel column chromatography, the eluent used is chloroform, obtain chloroform section, then mix the obtained chloroform section with polyamide, and use 70%- 100% methanol-water was used as the mobile phase to pass through the MCI column, and five components A to E were collected sequentially. The A (16.4g) component was subjected to silica gel column chromatography under gradient elution conditions, using petroleum ether / acetone as the The eluent was collected in sequence to obtain 5 fractions, whi...
Embodiment 2
[0047] The present invention dearomatizes isopentenylphloroglucinol derivatives hypatone A to Ca v 3.1 and mutant Ca v 3.1 (Arg1723His) Activation Activity Experimental methods and results are as follows:
[0048] 1. Cell preparation and expression
[0049] Human embryonic kidney (HEK) 293 cells were cultured in DMEM (HyClone) medium supplemented with 10% calf serum (Gibco) and double antibodies of penicillin (100 units / ml) and streptomycin (0.1 mg / ml) (Biological Industries) . 293 cells in the logarithmic growth phase were treated with LipoD293 TM (SignaGen Laboratories) transfection reagent mouse pCDNA3.1-Ca v 3.1, pCDNA3.1-Ca v 3.1 (Arg1723His) and pCDNA3.1-EGFP plasmids were transfected into cells. Transfected human embryonic kidney (HEK) 293 cells should be used within 48 hours.
[0050] 2. Electrophysiology experiment
[0051] All experiments were performed at room temperature (22-25°C). Using a microelectrode puller (P-1000, Sutter Instrument) and heating polis...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com