Synthesis of multimerized RGD peptide coupled Pyro photosensitizer and application thereof
A photosensitizer and coupling technology, applied in the field of photosensitizers, can solve the problems of poor tumor enrichment ability and insufficient affinity of integrin receptors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Embodiment 1. chemical synthesis
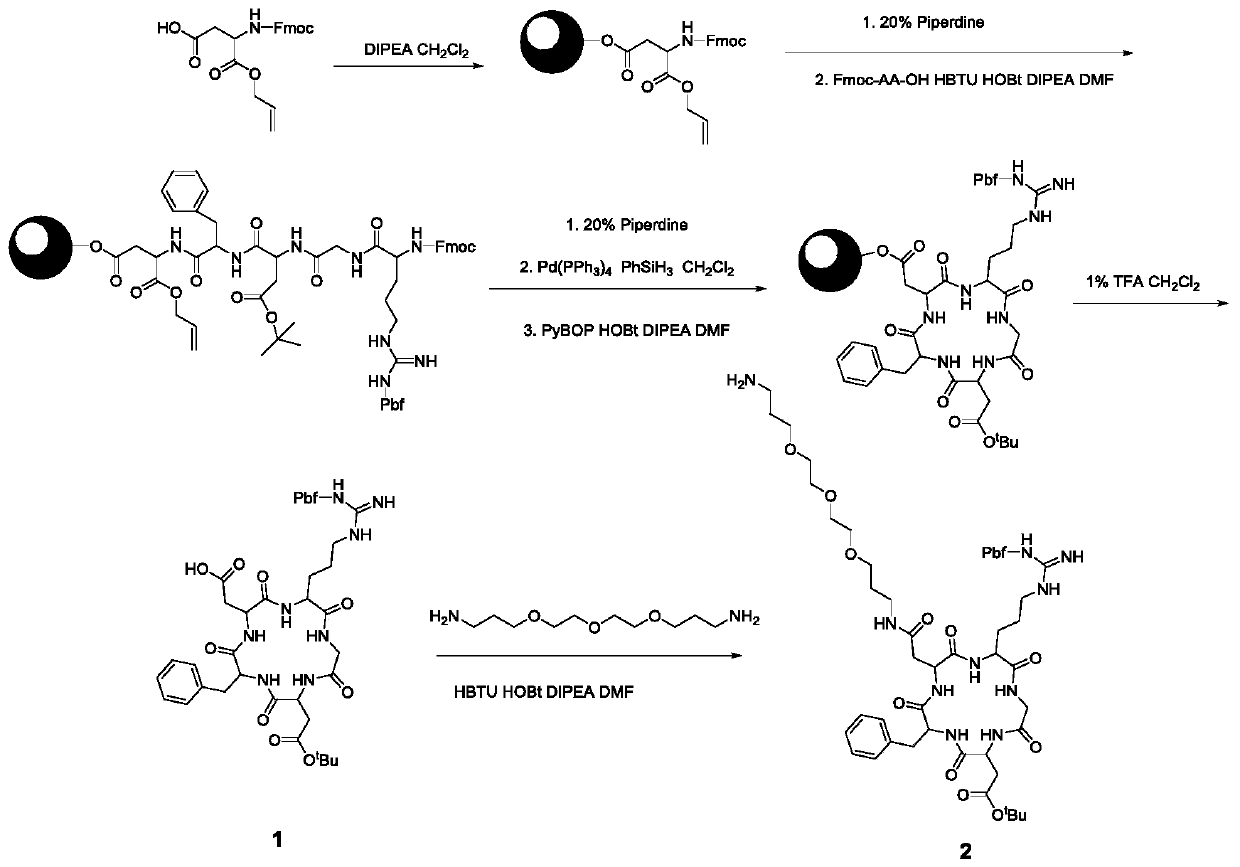
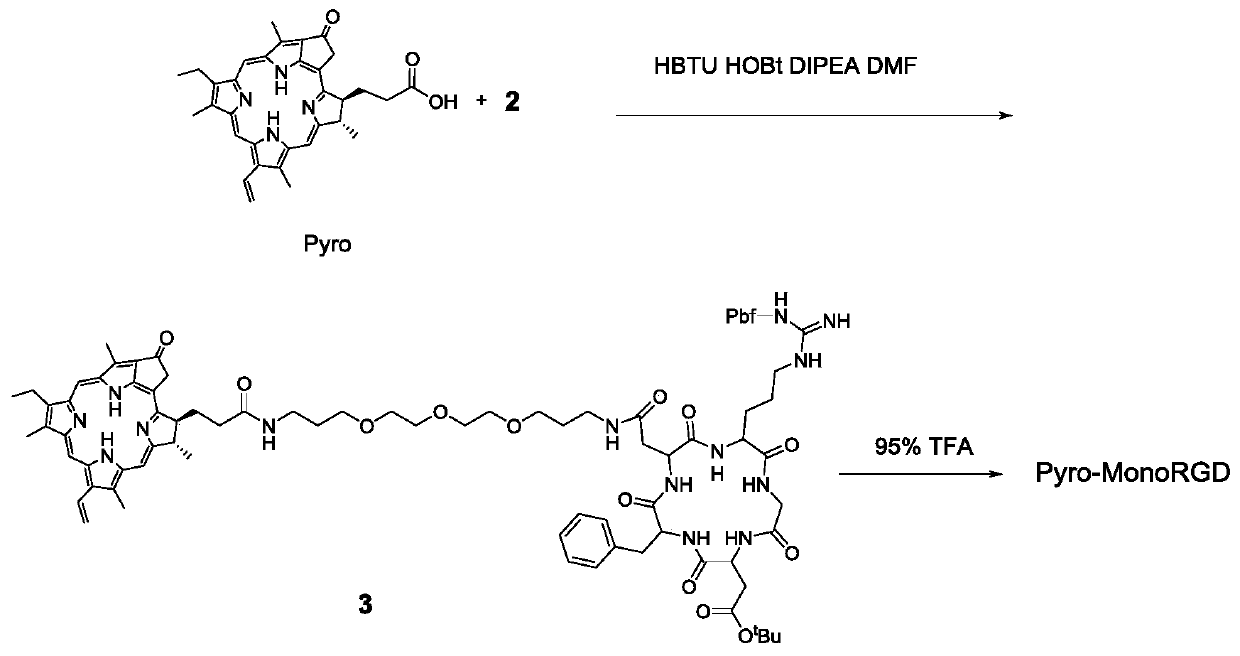
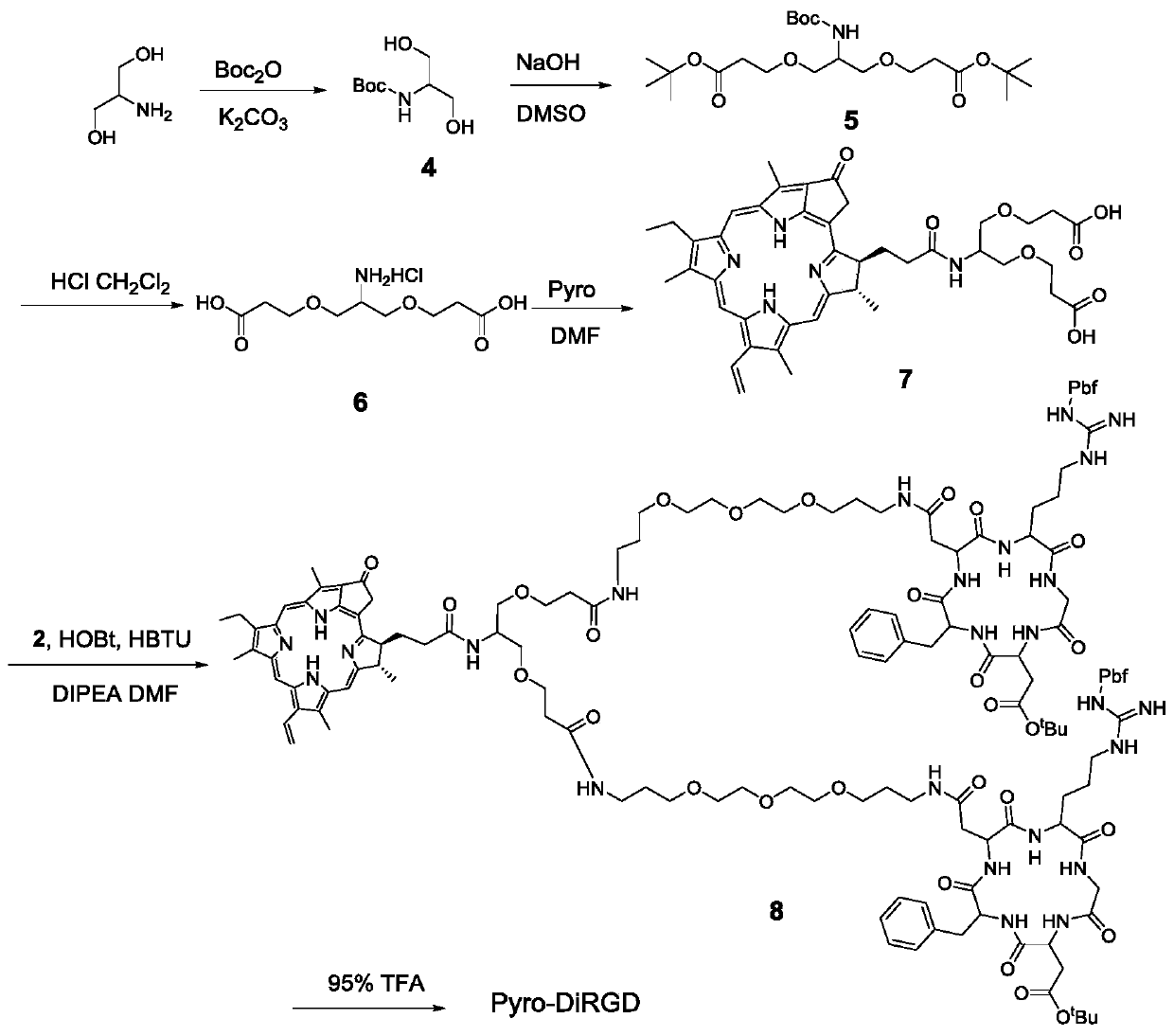
[0046] 1. Solid-phase synthesis of side chain-protected cyclic RGD pentapeptide 1 (attached figure 1 )
[0047] 2-Chlorotrityl chloride resin (8.0 g) in dichloromethane (CH 2 Cl 2 ) to swell in a sealed solid-phase synthesis tube for 1 h. Fmoc-Asp-OAll (949mg, 2.4mmol, 0.3mmol / g) and DIPEA (2.0mL, 5.0mmol) were dissolved in 30mL of dichloromethane, added to a solid-phase synthesis tube, and reacted for 5 hours. Then, using a blocking agent (CH 2 Cl 2 :MeOH:DIPEA=17:1:2, 20mL) to block the unreacted chlorine resin for 30 minutes. The Fmoc protecting group was then removed with 20 mL of 20% piperidine (PIP) in DMF for 30 minutes. Coupling of four amino acids was carried out using 2 eq of (Fmoc-D-Phe-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Gly-OH and Fmoc-Arg(Pbf)-OH) Fmoc protected derivatives respectively The reaction was reacted for 3 hours after in situ activation with HBTU (2.0 eq), HOBt (1.5 eq) and DIPEA (5 eq) in DMF. For each couplin...
Embodiment 2
[0072] Embodiment 2.Pyro-MonoRGD, Pyro-DiRGD, Pyro-TriRGD and Pyro are in the mensuration (table 1) of photophysical and photochemical property in DMSO solution
[0073] The UV-Vis absorption and fluorescence spectra of Pyro-MonoRGD, Pyro-DiRGD, Pyro-TriRGD and free Pyro in DMSO solution were measured. UV-Vis absorption spectra were recorded from 300 to 800 nm. Fluorescence emission and excitation spectra were recorded from 550 to 800 nm with excitation at 668 nm and emission at 672-673 nm using a Hitachi F-4500FL spectrophotometer. Using DPBF as a quencher and Pyro as a reference, the singlet oxygen quantum yields (ΦΔ) of Pyro-MonoRGD, Pyro-DiRGD and Pyro-TriRGD in DMSO solution were determined.
[0074] Table 1 The photophysical and photochemical parameters of Pyro-MonoRGD, Pyro-DiRGD, Pyro-TriRGD and Pyro in DMSO solution;
[0075]
[0076]The photophysical and photochemical properties of Pyro-MonoRGD, Pyro-DiRGD, Pyro-TriRGD and free Pyro are summarized in Table 1. A...
Embodiment 3
[0077] Example 3. Pyro-MonoRGD, Pyro-DiRGD and Pyro-TriRGD inhibit biotinylated vitronectin and α v beta 3 Receptor binding experiments (attached Figure 5 ,Table 2)
[0078] human integrin alpha v beta 3 (ITGAV&ITGB3) was diluted to 2.0 μg / mL in PBS buffer (pH 7.4), added to a 96-well plate (100 μL / well), and incubated overnight at 4°C. Then use 10% milk solution to seal the plate at 37°C for 2h, and then add different concentrations of (10 -3 to 10 -11 M) The three test compounds Pyro-MonoRGD, Pyro-DiRGD and Pyro-TriRGD were incubated at 37° C. on a shaker for 3 h. After washing, add streptavidin-HRP and incubate at 37°C for 1 hour. After washing again, incubate with 100 μL / well TMB single-component substrate solution for 30 minutes, and finally add 100 μL / well 2.0 M sulfuric acid to stop. Read the absorbance at 450 nm on a microplate reader. Each data point represents the average value of three replicate wells, and GraphPad Prism 5.0 software was used for data analy...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com