Rescue system for paramyxoviruses and rescue method for paramyxoviruses
A paramyxovirus and rescue system technology, applied in the field of viral reverse genetics, can solve problems such as the inability to meet the needs of paramyxovirus vaccine development, achieve good application prospects, and avoid pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1 Preparation of experimental cells and electroporation plasmids
[0052] Passage the Vero cells required for electroporation into a T75 flask at a ratio of 1:4, at 37°C, 5% CO 2 Culture for 24-48h until the cells are more than 70% confluent or a thin monolayer. After the Vero cells were digested with trypsin, an appropriate amount of medium (6-8 ml) was taken to elute, and 10 μl was sucked with a pipette for counting on a hemocytometer. After counting the cells, calculate the required amount of cells, and add the calculated amount of cells into a 1.5ml EP tube after autoclaving. Add the required plasmid into the 1.5ml EP tube after autoclaving according to the amount calculated in advance, and mark it.
[0053] Put the cells in the EP tube into a centrifuge, centrifuge at 2000g×5min at room temperature, and remove the culture medium. For each experimental sample, use 110 μl electroporation buffer to fully and gently suspend the cells, transfer the cell suspe...
Embodiment 2
[0054] Example 2 Exploration of Electroporation Conditions
[0055] Buffer: This system selects two electrotransfer buffers for experiments, AT electrotransfer buffer and BT electrotransfer buffer.
[0056] The AT electrotransfer buffer is Beijing Entranster-H electrotransfer buffer, catalog number: 98668.
[0057] BT electrotransfer buffer is lonzanucleofector electrotransfer buffer, catalog number: V4LP-3520.
[0058] Plasmid: pCDNA3.1-RFP with CMV promoter (can express red fluorescent protein in eukaryotic cells, which is convenient for later evaluation of electroporation efficiency by observing the expression of fluorescent protein);
[0059] Cells: Vero (the amount of cells used in each experimental group was 5×10 5 indivual).
[0060] Electrode Cup: C Manufacturer: Haimen Aibide Experimental Equipment Co., Ltd. QIVON Electrode Cup Item No.: CUV502
[0061] D Manufacturer: Bole Electric Rotor Cup Item No.: 165-2086.
[0062] Cells and plasmids were mixed and transfer...
Embodiment 3
[0070] Example 3 Rescue of recombinant measles virus MV-GFP by electroporation of whole plasmid in Vero cells
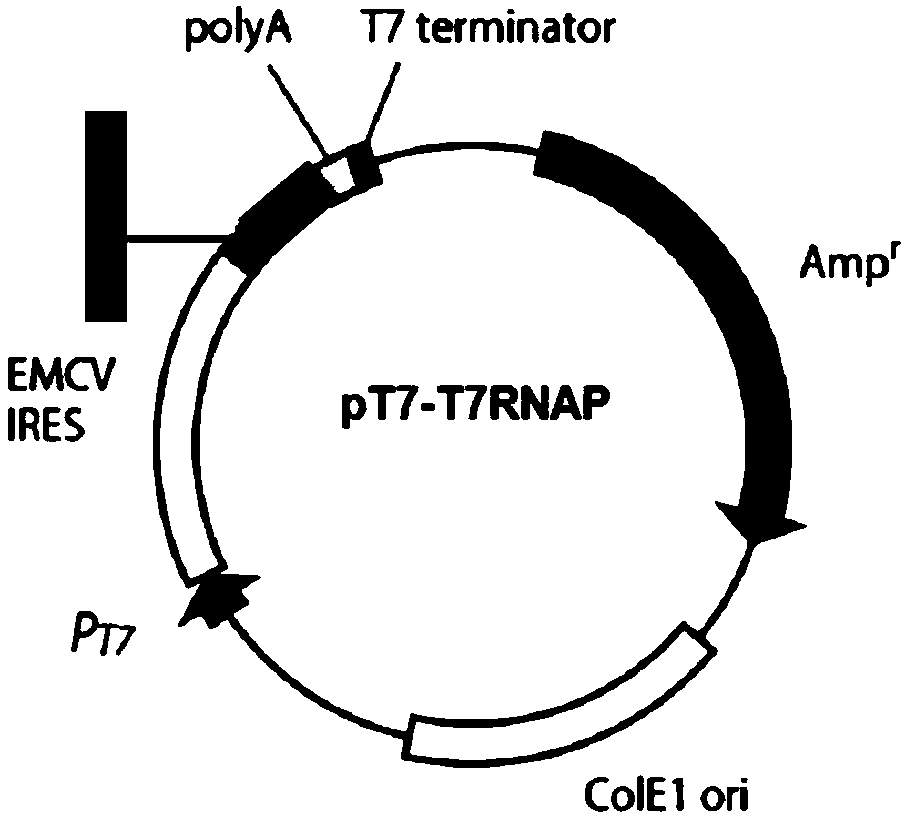
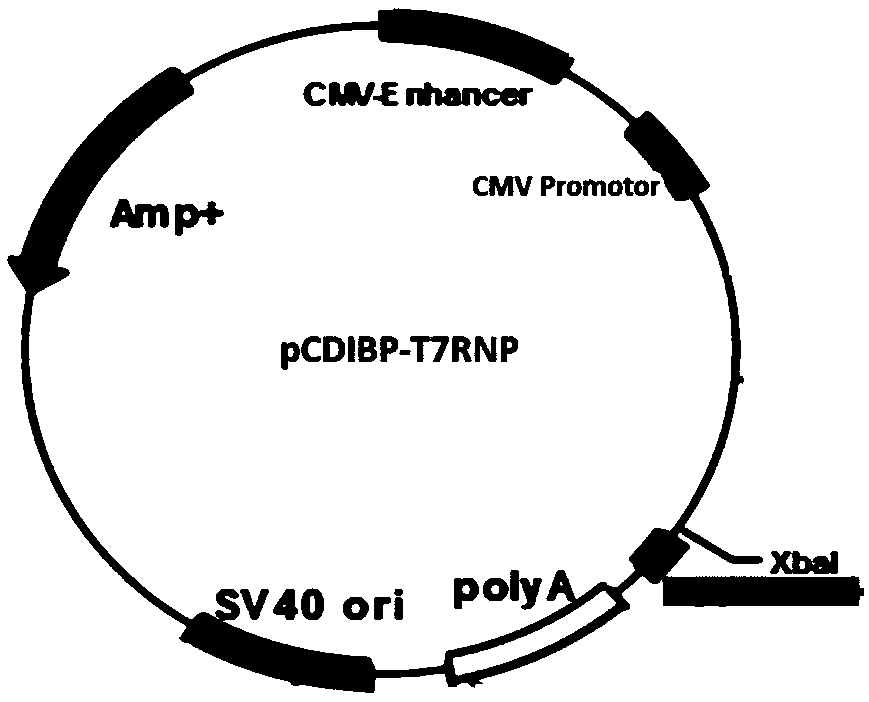
[0071] Plasmid used: pT7-MV S191 N. pT7-MV S191 P, pT7-MV S191 L, pCDIBP-T7RNAP (see SEQ ID NO.1 in the appendix for the sequence), pT7-T7RNAP (see SEQ ID NO.2 in the appendix for the sequence); pT7-MV S191 - For the construction of GFP, see patent CN201710001203 - a system and method for rescuing measles virus and recombinant measles virus.
[0072] Electrotransfer buffer: AT electrotransfer buffer; BT electrotransfer buffer.
[0073] Electric cup: D manufacturer.
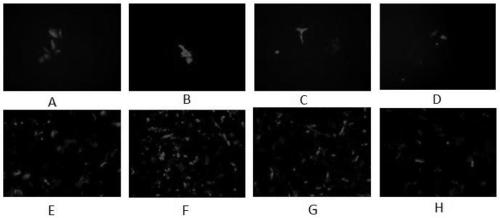
[0074] The experimental operation was carried out according to the optimal electroporation conditions explored in Example 1. The specific electroporation conditions and plasmid usage are shown in Table 3. If the test is successful, the resulting recombinant measles virus MV-GFP can express green fluorescent protein ( Figure 5 ) for later observation under a microscope.
[0075] Transfer the cells...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com